Abstract
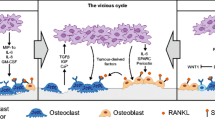
Hypercalcemia is a frequent complication of breast cancer which causes significant morbidity and mortality. Most commonly, it occurs in patients with multiple skeletal metastases. However, in a significant minority of patients, calcium levels become elevated in the absence of skeletal disease. In both instances, hypercalcemia is the result of pathological bone resorption caused by the secretion of cytokines that stimulate osteoclast differentiation and activity. One of these cytokines is parathyroid hormone-related protein (PTHrP). PTHrP is also secreted by normal breast cells during lactation to increase bone resorption and liberate skeletal calcium stores for the purposes of milk production. Therefore, the pathophysiology of hypercalcemia in breast cancer patients mimics the physiological processes that normally regulate calcium metabolism during lactation. Current therapy for hypercalcemia in breast cancer patients relies on the inhibition of bone resorption by a class of drugs known as bisphophonates. Newer therapies in development target cytokines involved in the recruitment and activation of osteoclasts by tumor cells.
Similar content being viewed by others
References
Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. Cancer statistics. CA Cancer J Clin 2004;54:8–29.
Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer 1987;55:61–6.
Scheid V, Buzdar AU, Smith TL, Hortobagyi GN. Clinical course of breast cancer with osseous metastasis treated with combination chemotherapy. Cancer 1986;58:2589–93.
Broadus AE. Mineral balance and homeostasis. In: Favus MJ, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism, 5th ed. Washington, DC: ASBMR; 2003. p. 105–11.
Juppner HW, Gardella TJ, Brown EM, Kronenberg HM, Potts JT. Parathyroid hormone and parathyroid hormone-related peptide in the regulation of calcium homeostasis and bone development. In: DeGroot LJ and Jameson JL, editors. Endocrinology, 4th ed. Philadelphia, PA: WB Saunders, 2001. p. 969–98.
LeBoff MS, Mikulec KH. Hypercalcemia: Clinical manifestations, pathogenesis, diagnosis, and management. In: Favus MJ, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism, 5th ed. Washington, DC: ASBMR, 2003. p. 225–30.
Mundy GR, Guise TA. Hypercalcemia of malignancy. Am J Med 1997;103:134–45.
Wysolmerski JJ, Broadus AE. Hypercalcemia of malignancy: The central role of parathyroid hormone-related protein. Ann Rev Med 1994;45:189–200.
Guise TA. Molecular mechanisms of osteolytic bone metastases. Cancer Suppl 2000;88:2892–8.
Guise TA, Mundy GR. Cancer and bone. Endocr Rev 1998;19:18–54.
Thomas RJ, Guise TA, Yin JJ, et al. Breast cancer cells interact with osteoblasts to support osteoclast formation. Endocinology 1999;140:4451–8.
Baron R. General principles of bone biology. In: Favus MJ editor. Primer on the metabolic bone diseases and disorders of mineral metabolism, 5th ed. Washington, DC: ASBMR. 2003. p. 1–8.
Philbrick WM, Wysolmerski JJ, Galbraith S, Holt E, Orloff JJ, Yang KH, Vasavada RC, Weir EC, Broadus AE, Stewart AF. Defining the roles of parathyroid hormone-related protein in normal physiology. Physiol Rev 1996;76:127–73.
Dunbar ME, Wysolmerski JJ. Parathyroid hormone-related protein: A developmental regulatory molecule necessary for mammary gland development. J Mammary Gland Biol Neoplasia 1999;4:21–34.
VanHouten JN, Dann P, Stewart AF, Watson CJ, Pollak, M, Karaplis AC. Wysolmerski JJ. Cre-mediated deletion of PTHrP from the mammary gland reduces bone turnover and preserves bone mass during lactation. J Clin Invest 2003;112:1429–36.
Powell GJ, Southby J, Danks JA, Stillwell RG, Hayman JA, Henderson MA, Bennet RC, Martin TJ. Localization of parathyroid hormone-related protein in breast cancer metastases: Increased incidence in bone compared with other sites. Cancer Res 1991;51:3059–61.
Vargas SJ, Gillespie MT, Powell GJ, Southby J, Danks JA, Mosley JM, Martin TJ. Localization of parathyroid hormone-related protein mRNA expression in breast cancer and metastatic lesions by in situ hybridization. J Bone Miner Res 1992;7:971–9.
Southby J, Kissan MW, Danks JA, Hayman JA, Moseley JM, Henderson MA, Bennett RC, Martin TJ. Immunohistochemical localization of parathyroid hormone-related protein in human breast cancer. 1990;50:7710–6.
Guise TA, Yin JJ, Taylor SD, Kumagai Y, Dallas M, Boyce BF, Yoneda T, Mundy GR. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J Clin Invest 1996;98:1544–9.
Guise TA, Yin JJ, Thomas RJ, Dallas M, Cui Y, Gillespie MT. Parathyroid hormone-related protein (PTHrP)-(1–139) isoforms is efficiently secreted in vitro and enhances breast cancer metastasis to bone in vivo. Bone 2002;30:670–6.
Hofbaeur LC, Heufelder AE. The role of receptor activator of nuclear factor-κB ligand and osteoprotegerin in the pathogenesis and treatment of metabolic bone diseases. J. Clin Endocrinol Metab 2000;85:2355–63.
Van Der Pluijm G, Sijmons B, Vloedgraven H, Deckers M, Papapoulos S, Lowik C. Monitoring metastatic behavior of human tumor cells in mice with species-specific polymerase chain reaction: Elevated expression of angiogenesis and bone resorption stimulators by breast cancer in bone metastases. J Bone Miner Res 2001;16:1077–91.
Sun B, Mitnick M, Yao G, Kacinsky B, Insogna K. Breast cancer-induced osteoclastogenesis: Possible roles for PTHrP and TNF-α. J Bone Miner Res 1998;13:S330.
Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Suda T, Shultz LD, Nishikawa S. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature 1990;345:442–4.
Felix R, Cecchini MG, Fleisch H. Macrophage colony stimulating factor restores in vivo bone resorption in the op/op osteopetrotic mouse. Endocrinology 1990;127:2592–4.
Bendre MS, Gaddy-Kurten D, Mon-Foote T, Akel NS, Skinner RA, Nicholas RW, Suva LJ. Expression of interleukin 8 and not parathyroid hormone-related protein by human breast cancer cells correlates with bone metastases in vivo. Cancer Res 2002;62:5571–9.
Stewart AF, Horst R, Deftos LJ, Cadman EC, Lang R, Broadus AE. Biochemical evaluation of patients with cancer-associated hypercalcemia: Evidence for humoral and nonhumoral group. N Engl J Med 1980;303:1377–83.
Burtis WJ, Wu T, Burch C, Wysolmerski JJ, Insogna KL, Weir EC, Broadus AE, Stewart AE. Identification of a novel 17,000 dalton parathryoid hormone-like adenylate cyclase-stimulating protein from a tumor associated with humoral hypercalcemia of malignancy. J Biol Chem 1987;262:7151–6.
Isales C, Carcangiu ML, Stewart AF. Hypercalcemia in breast cancer, reassessment of the mechanism. Am J Med 1987;82:1143–7.
Rogers MJ, Gordon S, Benford HL, et al. Cellular and molecular mechanisms of action of bisphosphonates. Cancer Suppl 2000;88:2961–78.
Russel RG, Rogers MJ. Bisphosphonates: From the laboratory to the clinic and back again. Bone 1999;25:97–106.
Saunders Y, Ross JR, Broadley KE. Systematic review of bisphosphonates for hypercalcemia of malignancy. Palliat Med 2004;18:418–31.
Wimalawansa SJ. Significance of plasma PTH-rP in patients with hypercalcemia of malignancy treated with bisphosphonate. Cancer 1994;73:2223–30.
Gurney H, Grill V, Martin TJ. Parathyroid hormone-related protein and response to pamidronate in tumor induced hypercalcemia. Lancet 1993;341:1611–3.
Walls J, Ratcliffe WA, Howell A, Bundred NJ. Response to intravenous bisphosphonate therapy in hypercalcemic patients with and without bone metastases: the role of parathyroid hormone-related protein. Br J Cancer 1994;70:169–72.
Wimalawansa SJ. Optimal frequency of administration of pamidronate in patients with hypercalcemia of malignancy. Clin Endocrinol 1994;41:591–5.
Major P, Lortholary A, Hon J, Abdi E, Mills G, Menssen HD, Yunus F, Bell R, Body J, Quebe-Fehling E, Seaman J. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol 2001;19:558–67.
Hortobagyi GN, Theriault RL, Porter L, Blayney D, Lipton A, Sinoff C, Wheeler H, Simeone JF, Seaman J, Knight RD, Heffernan M, Reitsma DK, Kennedy I, Allan SG, Mellars K. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. N Engl J Med 1996;335:1785–92.
Theriault RL, Lipton A, Hortobagyi GN, Leff R, Gluck S, Stewart JF, Costello S, Kennedy I, Simeone J, Seaman JJ, Knight RD, Mellars K, Heffernan M, Reitsma DJ. Pamidronate reduces skeletal morbidity in women with advanced breast cancer and lytic bone lesions: A randomized, placebo-controlled trial. J Clin Oncol 1999;17:846–54.
Hillner BE, Weeks JC, Desch CE, Smith TJ. Pamidronate in prevention of bone complications in metastatic breast cancer: A cost-effectiveness analysis. J Clin Oncol 2000;18:72–9.
Hillner BE, Ingle JN, Berenson JR, Janjan NA, Albain KS, Lipton A, Yee G, Biermann JS, Chlebowski RT, Pfister DG. American society of clinical oncology guideline on the role of bisphosphonates in breast cancer. J Clin Oncol 2000;18:1378–91.
Rosen LS, Gordon DH, Kaminski M, Howell A, Belch A, Mackey J, Apffelstaedt J, Hussein M, Coleman R, Reitsma DJ, Chen BL, Seaman JJ. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma. Cancer 2003;98:1735–44.
Hortobagyi GN. Novel approaches to the management of bone metastases in patients with breast cancer. Semin Oncol 2002;29:134–44.
Sato K, Onuma E, Yocum RC, Ogata E. Treatment of malignancy-associated hypercalcemia and cachexia with humanized anti-parathyroid hormone-related protein antibody. Semin Oncol 2003;30:167–73.
Oyajobi BO, Anderson DM, Traianedes K, Williams PJ, Yoneda T, Mundy GR. Therapeutic efficacy of a soluble receptor activator of nuclear factor κB-IgG Fc fusion protein in suppressing bone resorption and hypercalcemia in a model of humoral hypercalcemia of malignancy. Cancer Res 2001;61:2572–8.
Kovacs CS, Kronenberg HM. Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr Rev 1997;18:832–72.
VanHouten JN, Wysolmerski JJ. Low estrogen and high parathyroid hormone-related peptide levels contribute to accelerated bone resorption and bone loss in lactating mice. Endocrinology 2003;144:5521–9.
Thiede MA, Rodan GA. Expression of a calcium-mobilizing parathyroid hormone-like peptide in lactating mammary tissue. Science 1988;242:278–80.
Budayr A, Halloran BP, King JC, Diep D, Nissenson RA, Strewler GJ. High levels of a parathyroid hormone-like protein in milk. Proc Natl Acad Sci USA 1989;86:7183–5.
Sowers MF, Hollis BW, Shapiro B, Randolph J, Janney CA, Zhang D, Schork A, Crutchfield M, Stanczyk F, Russell-Aulet M. Elevated parathyroid hormone-related peptide associated with lactation and bone density loss. JAMA 1996;276:549–54.
VanHouten J, Dann P, McGeoch G, Brown EM, Krapcho K, Neville M, Wysolmerski JJ. The calcium sensing receptor regulates mammary gland production of PTHrP and calcium transport into milk. J Clin Invest 2004;113:598–608.
Sanders, JL, Chattopadhyay, N, Kifor, O, Butters, RR, Brown, EM. Extracellular calcium-sensing receptor expression and its potential role in regulating parathyroid hormone-related peptide secretion in human breast cancer cell lines. Endocrinology 2000;141:4357–64.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
DeMauro, S., Wysolmerski, J. Hypercalcemia in Breast Cancer: An Echo of Bone Mobilization During Lactation?. J Mammary Gland Biol Neoplasia 10, 157–167 (2005). https://doi.org/10.1007/s10911-005-5398-9
Issue Date:
DOI: https://doi.org/10.1007/s10911-005-5398-9