Abstract
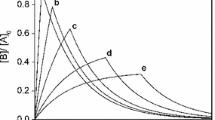
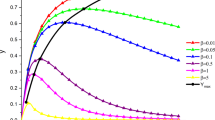
Analytical solutions are presented for four consecutive two-step kinetic schemes, which involve a first reaction with zeroth, first, second or mixed second order dependence and a second step, which is second order with respect to the intermediate formed in the first step. Two of the analytical solutions found use elementary functions not very commonly encountered in chemistry, as the rate equations are shown to be related to the Legendre or modified Bessel differential equations. The solutions are analyzed not only as a function of time, but by plotting two concentrations as a function of each other as well. The dependence of the kinetic traces on the parameter values is also investigated. In all cases, two scaling parameters are identified. Three of the four cases are characterized by a single shape parameter, which is basically the ratio of the rate constant scaled with a suitable concentration unit if necessary. The mixed second order–second order scheme has an additional shape parameter, which is the ratio of the initial concentrations of the two reactants.







Similar content being viewed by others
References
J.H. Espenson, Chemical Kinetics and Reaction Mechanisms, 2nd edn. (McGraw-Hill, New York, 1995)
P. Érdi, J. Tóth, Mathematical Models of Chemical Reactions (Manchester University Press, Manchester, 1989)
P. Érdi, G. Lente, Stochastic Chemical Kinetics. Theory and (Mostly) Systems Biological Applications (Springer, Heidelberg, 2014)
C.W. Gear, Commun. ACM 14, 176–179 (1971)
T.P.J. Knowles, C.A. Waudby, G.L. Devlin, S.I.A. Cohen, A. Aguzzi, M. Vendruscolo, E.M. Terentjev, M.E. Welland, C.M. Dobson, Science 326, 1533–1537 (2009)
F. Garcia-Sevilla, M. Garcia-Moreno, M. Molina-Alarcon, M.J. Garcia-Meseguer, J.M. Villalba, E. Arribas, R. Varon, J. Math. Chem. 50, 1598–1624 (2012)
D. Vogt, J. Math. Chem. 51, 826–842 (2013)
P. Miškinis, J. Math. Chem. 51, 1822–1834 (2013)
G. Milani, J. Math. Chem. 51, 2033–2061 (2013)
V. Vlasov, React. Kinet. Mech. Catal. 110, 5–13 (2013)
R.M. Torrez Irigoyena, S.A. Giner, J. Food Eng. 128, 31–39 (2014)
D.K. Garg, C.A. Serra, Y. Hoarau, D. Parida, M. Bouquey, R. Muller, Macromolecules 47, 4567–4586 (2014)
D. Belkić, J. Math. Chem. 52, 1201–1252 (2014)
A.A. Khadom, A.A. Abdul-Hadi, React. Kinet. Mech. Catal. 112, 15–26 (2014)
A. Izadbakhsh, A. Khatami, React. Kinet. Mech. Catal. 112, 77–100 (2014)
H. Vazquez-Leal, M. Sandoval-Hernandez, R. Castaneda-Sheissa, U. Filobello-Nino, A. Sarmiento-Reyes, Int. J. Appl. Math. Res. 4, 253–258 (2015)
J. Sun, D. Li, R. Yao, Z. Sun, X. Li, W. Li, React. Kinet. Mech. Catal. 114, 451–471 (2015)
G. Milani, T. Hanel, R. Donetti, F. Milani, J. Math. Chem. 53, 975–997 (2015)
G. Lente, J. Math. Chem. 53, 1172–1183 (2015)
M.L. Strekalov, J. Math. Chem. 53, 1313–1324 (2015)
G. Lente, Deterministic Kinetics in Chemistry and Systems Biology the Dynamics of Complex Reaction Networks (Springer, Heidelberg, 2015)
P. Muller, Pure Appl. Chem. 66, 1077–1184 (1994)
K.J. Laidler, Pure Appl. Chem. 68, 149–192 (1996)
http://mathworld.wolfram.com/ModifiedBesselFunctionoftheFirstKind.html
http://mathworld.wolfram.com/ModifiedBesselFunctionoftheSecondKind.html
http://mathworld.wolfram.com/LegendreFunctionoftheFirstKind.html
http://functions.wolfram.com/HypergeometricFunctions/LegendreQ3General/
J. Kalmár, É. Dóka, G. Lente, I. Fábián, Dalton Trans. 43, 4862–4870 (2014)
P.P. Levin, A.F. Efremkin, I.V. Khudyakov, Photochem. Photobiol. Sci. 6, 891–896 (2015)
N.D. Gomez, V. D’Accurso, V.M. Freytes, F.A. Manzano, J. Codnia, M.L. Azcárate, Int. J. Chem. Kinet. 45, 306–313 (2013)
G. Milani, F. Milani, J. Math. Chem. 51, 1116–1133 (2013)
G. Milani, A. Galanti, C. Cardelli, F. Milani, J. Appl. Polym. Sci. 131, 40075 (2014)
C. Brandt, I. Fábián, R. van Eldik, Inorg. Chem. 33, 687–701 (1994)
É. Dóka, G. Lente, I. Fábián, Dalton Trans. 43, 9596–9603 (2014)
Acknowledgments
The research was supported by the EU and co-financed by the European Social Fund under the Project ENVIKUT (TÁMOP-4.2.2.A-11/1/KONV-2012-0043). The author also thanks the Hungarian Science Foundation for financial support under grant No. NK 105156.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lente, G. Analytical solutions for the rate equations of irreversible two-step consecutive processes with second order later steps. J Math Chem 53, 1759–1771 (2015). https://doi.org/10.1007/s10910-015-0517-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10910-015-0517-3