Abstract
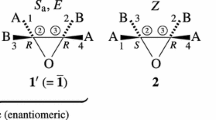
The stereoisogram approach (Fujita in J Org Chem 69:3158–3165, 2004, Tetrahedron 62:691–705, 2006, Tetrahedron 65:1581–1592, 2009) is applied to trigonal bipyramidal compounds, where chiral and achiral proligands are taken into consideration. After configurations of trigonal bipyramidal compounds are enumerated by using the partial-cycle-index method (Fujita in Symmetry and combinatorial enumeration in chemistry. Springer, Berlin, 1991), they are categorized into Type I–V cases according to the stereoisogram approach. The enumerated configurations are specified by configuration indices and C/A-descriptors, which are assigned in terms of RS-diastereomeric relationships, (not of enantiomeric relationships). The concept of a multiplet of stereoisograms is proposed to formulate the concept of ortho-stereogenicity, which is concerned with ortho-diastereomeric relationships between stereoisograms. On the other hand, the concept of stereogenicity (which has been used in the conventional stereochemistry) is redefined by starting from RS-stereogenicity and by comparing with the ortho-stereogenicity, where the stereogenicity is concerned with diastereomeric relationships between pairs of enantiomers. Berry’s pseudorotation for isomerization of trigonal bipyramidal compounds is reinterpreted in order to cover more general cases in which chiral moieties along with achiral moieties (i.e., all of Type I–V cases) are taken into consideration. A modified Desargues-Levi graph is proposed to cover Type I–V cases. In addition, an adamantane-like graph is proposed to formulate Berry’s pseudorotation on the basis of a multiplet of stereoisograms, where quadruplets of RS-stereoisomers occupy the nodes of the graph. Thereby, a multiplet of stereoisograms is shown to be a versatile tool to characterize stereoisomerization processes of inorganic stereochemistry in addition to those of organic stereochemistry.
Similar content being viewed by others
References
Berry R.S.: J. Chem. Phys. 32, 933–938 (1960)
Muetterties E.L.: J. Am. Chem. Soc. 91, 1636–1643 (1969)
Muetterties E.L.: J. Am. Chem. Soc. 91, 4115–4122 (1969)
Ugi I., Marquarding D., Klusacek H., Gillespie P., Ramirez F.: Acc. Chem. Res. 4, 288–296 (1971)
Brocas J., Gielen M., Willem R.: The Permutational Approach to Dynamic Stereochemistry. McGraw-Hill International, New York (1983)
Ugi I., Dugundji J., Kopp R., Marquarding D.: Perspectives in Theoretical Stereochemistry. Springer, Berlin (1984)
Wood J.S.: Prog. Inorg. Chem. 16, 227–486 (1972)
Ugi I.: Chimia 40, 340–350 (1986)
IUPAC Organic Chemistry Division, Pure Appl. Chem. 68 2193–2222 (1996)
Engel R., Rizzo J.I.: Curr. Org. Chem. 10, 2393–2405 (2006)
Swamy K.C.K., Kumar N.S.: Acc. Chem. Res. 39, 324–333 (2006)
Lauterbur P.C., Ramirez F.: J. Am. Chem. Soc. 90, 6722–6726 (1968)
Balaban A.T., Farcasiu D., Banica R.: Rev. Roum. Chim. 11, 1205–1227 (1966)
Balaban A.T.: J. Chem. Inf. Model. 35, 339–350 (1995)
DeBruin K.E., Naumann K., Zon G., Mislow K.: J. Am. Chem. Soc. 91, 7031–7040 (1969)
Mislow K.: Acc. Chem. Res. 3, 321–331 (1970)
Randić M.: Int. J. Quantum Chem. 15, 663–682 (1979)
Randić M., Katović V.: Int. J. Quantum Chem. 21, 647–663 (1982)
Couzijn E.P.A., Slootweg J.C., Ehlers A.W., Lammertsma K.: J. Am. Chem. Soc. 132, 18127–18140 (2010)
Moberg C.: Angew. Chem. Int. Ed. Engl. 50, 10290–10292 (2011)
Hellwinkel D.: Chem. Ber. 99, 3642–3667 (1966)
Kojima S., Kajiyam K., Akiba K.: Tetrahedron Lett. 35, 7037–7040 (1994)
Hou J.-B., Zhang H., Guo J.-N., Liu Y., Xu P.-X., Zhao Y.-F., Blackburn G.M.: Org. Biomol. Chem. 7, 3020–3023 (2009)
Yang G., Xu Y., Hou J., Zhang H., Zhao Y.: Dalton Trans. 39, 6953–6959 (2010)
Fujita S.: J. Org. Chem. 69, 3158–3165 (2004)
Fujita S.: J. Math. Chem. 35, 265–287 (2004)
Fujita S.: MATCH Commun. Math. Comput. Chem. 52, 3–18 (2004)
Fujita S.: MATCH Commun. Math. Comput. Chem. 61, 11–38 (2009)
Fujita S.: Tetrahedron 62, 691–705 (2006)
Fujita S.: Tetrahedron 65, 1581–1592 (2009)
Fujita S.: J. Comput. Aided Chem. 10, 16–29 (2009)
Fujita S.: J. Math. Chem. 49, 95–162 (2011)
Fujita S.: Symmetry and Combinatorial Enumeration in Chemistry. Springer, Berlin (1991)
Fujita S.: Bull. Chem. Soc. Jpn. 63, 1876–1883 (1990)
Connelly N.G., Damhus T., Hartshorn R.M., Hutton A.T.: Nomenclature of Inorganic Chemistry. IUPAC Recommendations 2005. The Royal Society of Chemistry, Cambridge (2005)
Fujita S.: Theor. Chim. Acta 76, 247–268 (1989)
Fujita S.: J. Math. Chem. 5, 121–156 (1990)
Fujita S.: Bull. Chem. Soc. Jpn. 63, 203–215 (1990)
Fujita S.: J. Math. Chem. 12, 173–195 (1993)
Fujita S.: Bull. Chem. Soc. Jpn. 73, 329–339 (2000)
Fujita S.: Theor. Chim. Acta 82, 473–498 (1992)
Fujita S.: Bull. Chem. Soc. Jpn. 63, 315–327 (1990)
Fujita S.: Tetrahedron 47, 31–46 (1991)
Fujita S.: Tetrahedron 60, 11629–11638 (2004)
von Zelewsky A.: Stereochemistry of Coordination Compounds. Wiley, Chichester (1996)
Eliel E.L., Wilen S.H.: Stereochemistry of Organic Compounds. Wiley, New York (1994)
Eliel E.L., Willen S.H., Doyle M.P.: Basic Organic Stereochemistry. Wiley-Interscience, New York (2001)
Prelog V., Helmchen G.: Helv. Chim. Acta 55, 2581–2598 (1972)
S. Fujita, in Carbon Bonding and Structures. Advances in Physics and Chemistry, ed. by M.V. Putz, (Springer, Dordrecht, 2011), vol. 5 of Carbon Materials: Chemistry and Physics, Chapter 10, pp. 227–271
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fujita, S. Stereoisograms of trigonal bipyramidal compounds: II. RS-stereogenicity/RS-stereoisomerism versus stereogenicity/stereoisomerism, leading to a revised interpretation of Berry’s pseudorotation. J Math Chem 50, 1815–1860 (2012). https://doi.org/10.1007/s10910-012-0007-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10910-012-0007-9