Abstract
The synergy between graphene and conducting polymers has the potential to revolutionize the energy storage sector to a more dependable, sustainable, and affordable energy source. Introducing graphene nanoparticles in the conductive polymers (polypyrrole and polythiophene) nanoparticles is a prospective technique to increase the charge transfer efficiency of the resulting nanocomposite. Subsequently, the fabrication method of graphene-polymer nanoelectrode is the most critical factor responsible for their excellent performance. This review presents a concise summary of graphene (Gr), polypyrrole (PPy), and polythiophene (PTh) synthesis techniques. The study revealed that the dispersion of nanoparticles could be controlled by suitable solvent, mixing approach, and drying conditions. In addition, the PPy/PTh/Gr nanocomposite is envisaged to be a promising nanoelectrode for sustainable and efficient energy storage capabilities. The future approaches to developing improved materials synthesis techniques for multi-applications (supercapacitors, sensors, and photovoltaic) are also provided.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In the current dispensation, the impasses experienced by fossil fuel driving systems negatively affect both developed and developing countries. By considering the dependability of technology on the non-interruption of power supply in the medical, transportation, aviation, agricultural, and security systems, the development of reliable energy supplies to these critical sectors and many other sectors cannot be overstated. Hence, it calls for unwavering and dedicated attention. The continual rise in the global population is directly related to the rise in energy demand, which, in turn, increases the challenges of non-renewable energy sources. Besides the menaces of non-renewable energy sources, such as global warming and environmental pollution, the depleting condition of petroleum resources threatens the continued global need for energy [1]. Therefore, the convincing exploration of renewable energy to reduce global warming, the projection of positive environmental impacts, and stable climatic conditions are valuable for the energy paradigm transition. However, intermittent renewable energy output directly impacts automobile and grid systems (system collapse, voltage, and frequency fluctuations) [2]. One way to solve the intermittent challenges of renewable energy is to provide an efficient storage system and possibly electrochemical energy storage.
However, electrochemical energy storage has challenges like cost, durability, sustainability, and efficiency problems. These problems are often associated with the materials employed to fabricate electrochemical electrodes [3]. For instance, lithium-ion batteries have relevance in portable devices. However, their applications for powering electric vehicles still need to be investigated. Lithium-ion batteries’ reliability, specific energy, and power density negate their merits. These include weight, flexibility, low cost, low self-discharge, and the absence of memory effect, which require prompt attention in developing new electrodes for lithium-ion batteries. Graphite, carbon nanotubes, and graphene are typical anode materials for lithium-ion batteries. Usually, the poor power density of lithium batteries arises from the poor ionic transition in the anode electrodes. Amongst the anode materials, graphene and its derivatives and composites with graphene and graphene derivatives have shown better electrochemical, thermal, mechanical, electrical, and flexibility properties [4, 5].
Graphene is a carbonaceous material with excellent electrical conductivity, tunable functional ability, and mechanical properties. The unique properties of graphene and graphene-based materials qualify them as potential materials for energy storage. In addition, graphene synthesis methods’ simplicity, flexibility, and cost-friendliness are other qualities of graphene for energy storage materials [6]. However, graphene and graphene-related materials have band gaps, which limit their practical and commercial applications [7]. These challenges can be overcome by compositing graphene and its other nomenclatures with conjugated polymers. In this case, the synthetic methods of graphene play a significant role.
Polythiophene and polypyrrole are conjugated-conductive polymers with high electrical conductivity with outstanding applications in energy storage, solar cell devices, organic-transistor, sensors, and coating [8]. The properties of conjugated-conductive polymers can be easily manipulated using structural modifications and synthesis methods to route the materials to the desired applications. The doping process, which involves introducing “impurities/foreign” materials into the intrinsically conjugated polymer, is another means of controlling the properties of the polymers. Polythiophene and polypyrrole are conjugated-conducting polymers with high charge-carrier mobility, environmentally benign, low cost, good electrical conductivity, and long-wavelength absorption. Nonetheless, the pseudo-capacitive effects of polypyrrole and polythiophene are barriers to their long-discharge stability. Hence, to resolve the associated problems of graphene, polythiophene, and polypyrrole, the hybrid formation of polythiophene/polypyrrole-loaded graphene (PTh/PPy/Gr) nanocomposite is envisaged to be a promising electrochemical energy storage electrode, wherein some scientific investigations have been documented in this domain.
For instance, using the interfacial polymerization synthesis method, Bora et al. [9] studied the electrical and electrochemical characteristics of PTh/graphene oxide. Improvements in the thermal, electrical properties, and electrochemical reversibility were recorded for the PTh/graphene oxide composite. Graphene oxide (GO) is a graphene family produced by chemical exfoliation and the oxidization of layered-crystalline natural or artificial graphite [9]. Li et al. reported an enhanced electrochemical performance, high energy/power density, and good cyclability for the composite of PTh/GO [10]. The electrochemical performances of the fabricated PTh/GO composites were attributed to the uniformity in the structure and the interfacial interaction of PTh and GO; this scenario is a function of the synthesis methods.
Wang et al. [11] employed a facile, one-step-hydrothermal synthesis method to produce the PPy/graphene aerogel composite with 3-dimensional porous channels. The fabricated PPy/graphene aerogel demonstrated high specific capacitance, energy/power density, and excellent cyclability with the needed flexibility. Ye et al. [11] experimental results on the fabrication of PPy/graphene-expanded aerogel composite showed the possibility of improving the performances of polypyrrole for energy storage applications by adding graphene. In the investigation by Kong et al. [12] a 2-facile electrochemical step was used to fabricate the composite of a bowl-shaped graphene-loaded PPy. The reported unique electrochemical performances of the composite were attributed to the reduction in the capacitance degradation and enhanced rate of implementation of the composite. The hybrid of two conducting polymers (polypyrrole and polyaniline), loaded-graphene-oxide,was carried out by Zhao et al. [13], using synthetic interfacial methods, viz: vapour/liquid interaction reaction. The fabricated composite exhibited excellent electrochemical properties, high capacity, and stability. Several authors have explored different techniques in this topical field of study [14,15,16].
The prospect of the hybrid formation of graphene nanoparticles and conductive polymers (polythiophene/polypyrrole) in energy storage applications aligns with the aspiration for a solution to the challenges in electrochemical energy storage, and their merits are reasonably promising. This has led to the growing body of literature focusing on this exciting subject [17,18,19,20,21,22]. Based on these and as a way of developing an improved technique, it becomes necessary to examine the existing methods and their associated technical challenges. Therefore, this study presents the scientific investigations on the hybrid polypyrrole/polythiophene-loaded graphene for energy storage applications.
The study, specifically, revealed the characteristics of the individual material and their nanocomposites for energy storage applications. Notably, beyond all factors, which influence polymers and their nanocomposite performances for electrochemical energy storage applications, are their synthesis methods, which are systematically investigated in this review. Polymer nanocomposites’ electrical, mechanical, chemical, cost, electrochemical, and environmental impact properties depend on their synthesis techniques and are thoroughly examined and presented. The present study, the first of its kind, provides brief evidence of the potential and promising status of graphene/conducting polymer nanocomposites. In addition, the study has benefits in manufacturing polymer-based nanocomposites for electrochemical energy storage.
2 Synthesis of Polythiophene, Polypyrrole, and Graphene
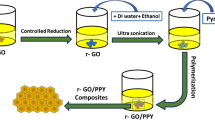
The conditions and characteristics of materials’ intrinsic and extrinsic properties are usually controlled and manipulated by suitable synthesis techniques; hence, the synthesis method determines the application areas of the synthetic composite materials. The morphology, purity, crystallinity, and the various characteristics of materials and their composites for the desired applications are determined by choice of synthetic methods [23]. Factors affecting synthesis include temperature, time, size, porosity, environmental factor, pH value, pressure, substrate interaction, etc. [24]. Therefore, surveying the available synthesis methods to produce polythiophene, polypyrrole, and graphene is imperative to fabricate reliable and efficient energy storage electrodes (Fig. 1).
2.1 Fabrication of Polythiophene and Polythiophene Nanocomposites
Polythiophene is a conducting polymer with applications for manufacturing sensors, supercapacitors, batteries, and anti-corrosion of metals. This is due to conducting polymers’ unique electrical, chemical, magnetic, thermal, and high electron mobility properties. The simplicity of polythiophene’s fabrication methods, such as chemical-oxidative, solid state, metal-catalyzed coupling reaction, acid-catalyzed, and electro-polymerization, have been considerably reported [25]. However, it was reported by Lu et al. [25] that some of the methods mentioned earlier are responsible for the rigid backbone and \(\pi\)-conjugation pattern of polythiophene therefore poor solubility in organic solvents,ad deficient processability limits its practical applications. A cationic-surfactant polymerization of thiophene in the presence of ferric chloride was performed by Gnanakan et al. [26]. The preparation procedures follow the dissolving of 1.0 g of thiophene monomer and 0.0034 mol of cetrimonium bromide surfactant in 30 ml of distilled water. Ferric chloride was added to the thiophene surfactant solution after 15 min of stirring; the resultant solution was allowed to polymerize at room temperature for 24 h under continuous stirring. The filtrated polythiophene residue was dried for 6 h 80 °C. The polythiophene’s electrochemical performance displayed the following characteristics: 134 F/g, 8 Wh/kg, and 396 W/kg specific capacitance, energy, and power density, respectively.
Pascariu et al. [27] investigated the electrical properties of PTh/nickel nanocomposite fabricated via the electrochemical oxidative polymerization method. For 2 min, Pascariu et al. electrochemically polymerized thiophene in a potentiostat–galvanostat instrument in a 7 ml of acetonitrile with 1.9 mM thiophene and some measured quantities of nickel. Figure 2 shows the morphology, chemical structure, and density properties of the PTh/nickel nanocomposite fabricated by Pascariu et al. As shown in Fig. 2, the PTh is granular in shape with uniform distribution of nickel on its surface. Figure 2b. shows that higher nickel results in decline Raman intensity, which is consistent with earlier research [28, 29]. Additionally, the symmetric in-phase vibration of thiophene rings diffusing across the polymer chain may cause an intense band at about 1455 cm−1. According to the authors, an increase in Ni concentration affects the active surface area leading to better electrochemical properties. Due to this, the maximum specific capacitance of 3000 Fg−1 and quick discharging process were observed. When composited with viable electrochemical material, tusingpolythiophene as an energy storage electrode is realizable if a proper synthetic approach is adopted to produce the composite electrode [30, 31]. Gin et al. [32] prepare a PTh-diode by electro-polymeelectro-polymerizing thiophene and controlled electric potentialnion doped on both sides. The authors reported that the fadiode was flexible and more robust than some metals, e.g., aluminum. Modifying electrodes by electro-polymerizing thiophene is useful for voltametric sensors of some ovoltammetricbiological molecules. In addition, PTh-loaded nanomaterials are helpful for electrochemical sensors with low-detection limits, reasonable sensitivity, and high stability/reproducibility [33].
PTh/nickel a, b morphology, c chemical structure, and d density properties Reproduced with permission from Ref [27]
A supercapacitor-based electropolymerized-PTh/multiwalled carbon nanotube composite was prepared by Thakur et al. [34]. The in-situ chemical oxidation polymerization of the PTh/carbon-nanotube nanotube follows the addition of 1/5 g of carbon nanotube in 50 ml of chloroform. 12 g of iron chloride was added to the sonicated solution of ccarbon nanotube 1.0 g of thiophene was added to the mixture and stirred for − 15 h. The resultant precipitate was, after centrifugation, dried at 80 °C. Figure 3 shows the structural, cyclic voltammetry, morphology, and galvanostatic properties of the PTh/carbon-nanotube nanocomposite. As shown in Fig. 3, the amorphous nature and the shift in the diffraction peak of the PTh from a diffraction angle, 2θ of 15°–25°, was observed. The morphology shows a well-dispersed nanoparticle within the interface of the PTh polymer. Moreso, the PTh/nanoparticle-based composite’s current density increased as the carbon nanotube’s volume increased.
PTh/carbon-nanotube a structural, b cyclic voltammetry, c morphology, and d galvanostatic charge/discharge properties. Reproduced with permission from Ref. [34]. ©2016, IOP Publishing
PTh/aluminum-oxide nanocomposite was investigated by Vijeth et al. [35] by using an in-situ chemical polymerization. The PTh/aluminum-oxide nanocomposite electrode was prepared using camphor-sulfonic acid in an 8% aluminum oxide (Al2O3). The supercapacitive behavior and the specific capacitance of the PTh and PTh/aluminum oxide nanocomposites are displayed in Fig. 4a–c. The aluminum oxide can improve the specific capacitance of the PTh from 655 to 757 F/g. In Fig. 4c, the structural property of the PTh and its composite with aluminum oxide showed an amorphous peak at 2θ = 21.9°. However, a downward shift and narrow peak were observed for the PTh and a sharp rise at 67.8° for the aluminum oxide.
The PTh/aluminum nanocomposite properties a, b PTh and PTh/aluminum cyclic voltammograms c PTh and specific capacitance d structural plane. Reproduced with permission Ref. [35]. ©2018, American Institute of Physics
Due to the limitations of aluminum-ion batteries in the presence of carbon electrodes, Kong et al. [36], fabricated the nanocomposite of PTh and graphene oxide for an aluminum-ion cathode electrode. The PTh/graphene oxide nanocomposite was reported to possess excellent conductivity, a specific capacity of ~ 130 mAh/g, a good capability to retain charge at the constant capacity of 100 mAh/g after 4000-cycles and 86 mAh/g at a current density of 5000 mA/g. As shown in Fig. 5a, Kong et al. employed the oxidative chemical polymerization procedure to fabricate the PTh/graphene oxide nanocomposite. The stability of polymers in the two-dimensional nanomaterials, and low-cost and simple synthesis methods are among the benefits of the chemical oxidative polymerization method. The specific capacity (Fig. 5b) experienced an incremental behavior as the cut-off potential increased from 2.2 to 2.45 V. However, the Coulombic efficiency, which depends on the decomposition of battery electrolyte, decreased with an increase in the cut-off potential. The Coulombic and energy efficiencies are essential parameters that must be calculated while determining the properties of nanoelectrodes [26]; the difference between the two efficiencies is highlighted by Eq. 1. The internal resistance of active materials determines, to a large extent, the specific capacity of nanoelectrode and its particular energy.
where \(Q_{f}\) is the Coulomb efficiency, \(D_{t}\) is the discharge time, \(C_{t}\) is the charge time, \(E_{f}\) is the energy efficiency, \(SPE_{D}\) is the specific energy at discharge and \(SPE_{C}\) is the specific energy at a charge.

Reproduced with permission from Ref. [36]. ©2020, American Chemical Society
PTh/graphene-oxide a chemical oxidative polymerization method b specific capacity.
Polythiophene’s classic properties, such as good electrical conductivity, electrochemical, simple polymerization method, thermal stability, and environmental stability, have paved the way for research into its various promising application areas. Nevertheless, pristine PTh’s performances require further enhancement by including suitable materials. PTh-based nanocomposites are used as sensors, photosensitizers, electromagnetic inference, and electrochemical electrodes [37, 38].
In a different application domain, a PTh-based nanocomposite was developed by Haghgir et al. [39] to remove wastewater pollutants. Pollution because of industrial waste can be very damaging to the existence of human beings and animals. Haghgir et al. employed a chemical polymerization method to prepare PTh/zeolite/magnetic iron nanocomposite. The nanocomposite’s high pollutant absorbance efficiency was achieved at 50 and 25 wt.% of zeolite and magnetic iron in the polymer matrix. The highest adsorption capacity of –319 mg/g at 80 °C was achieved. A solution mixing method, known as the liquid/liquid interfacial polymerization method, was carried out by Hellmann et al. [40] to prepare a nanocomposite of PTh/gold and PTh/gold/carbon nanoparticles. The inclusion of carbonaceous materials in polymers is an excellent way to modify their electrical conductivity and charge transfer characteristics for desirable applications. Hellmann et al. described the visibility of PTh-based nanocomposite as active layers in photovoltaic devices. The introduction of carbon nanotube in the PTh/gold nanoparticles was reported to have increased the charge transfer efficiency of the nanocomposite. More so, the bandgap of the PTh/gold nanoparticles was reduced by a moderate inclusion of carbon nanotube nanoparticles.
As a gas sensor, Polythiophene-based nanocomposite was reported in a short communication by Amruth et al. [41]. In general, nanomaterials and their composites have the advantages of a high surface-to-volume ratio, simple fabrication methods, and excellent gas sensor sensitivity. For this purpose, Amruth et al. carried out an in-situ polymerization of PTh/chitosan nano-power. Chitosan was dispersed in chloroform containing thiophene monomers, and ferric chloride was added to the agitated solution under rigorous stirring. The residue of the resultant solution was washed using methanol, chloroform, and water and later dried at 50 °C for 24 h. The microstructure, electrical conductivity, and sensitivity properties of the PTh/chitosan nanocomposite are shown in Fig. 5. The electrical conductivity of the composite was influenced mainly by the synthesis method employed. The well-dispersed nanoparticles with the chitosan power (i.e., homogenous mixture) yielded a better conductive property. The PTh/chitosan composite, which was subjected to ammonia gas, responded to the gas sensing at room temperature. Furthermore, the photocatalytic and pathogen control capabilities of the PTh-nanocomposite were excellent (Fig. 6).

Reproduced with permission from Ref. [41]. ©2022, Elsevier Ltd
PTh/chitosan nanocomposites a microstructure b electrical conductivity and c gas sensitivity properties.
Noreen et al. [42] fabricated the nanocomposite of PTh/graphene nanoplatelet using the oxidative chemical polymerization method. The nanocomposite was applied for the photo-catalytic degradation of bromophenol blue and pathogen control. The structural property of the nanocomposite (Fig. 7a) revealed its formation by Van der Waals force, and as the graphene filler increased, the X-ray peak intensity of the nanocomposite increased. The photocatalytic degradation efficiency (Fig. 7b and d) of the nanocomposites was observed to have increased as the graphene content increased in the polymer matrix. As shown in Fig. 7c, the pathogen activities of the nanocomposites exhibited the highest performances against microbes at the highest concentration of filler content.

Reproduced with permission from Ref. [42]. ©2022, Elsevier Ltd
PTh/graphene-nanoplatelet nanocomposite a structural b photocatalytic degradation c pathogen control and d absorbance properties.
Furthermore, the effect of platinum and reduced graphene oxide nanoparticles on PTh were investigated by Tsai et al. [43]. The PTh/platinum/reduced graphene-oxide nanocomposite was fabricated using a one-pot redox synthesis method. The one-pot synthesis method is a one-reaction method, which is viable for the manufacturing of organic/inorganic composite materials [44]. The fabrication procedures and the application of the PTh/platinum/reduced graphene-oxide nanocomposite is shown in Fig. 8. As a humidity sensor, the PTh/platinum/reduced graphene-oxide was characterized by excellent flexibility, electrical conductivity, high sensitivity, and linearity properties.

Reproduced with permission from Ref. [43]. ©2020, Elsevier Ltd
PTh/platinum/reduced graphene-oxide nanocomposite fabrication procedures and application area.
The plurality of research on PTh-based nanoparticles and their nanocomposites for energy storage, sensors, and photocatalytic applications have shown the excellent functionalities of PTh in diverse ways. Li et al. employed an esterification reaction method for producing PTh/graphene-oxide for energy storage applications [10]. The PTh-nanocomposite yielded a 971 F/g, 66.1 Wh/kg, and 7000 W/kg specific capacitance, energy, and power densities, respectively, while capacitance stability of ~ 98% can be achieved within 10,000 cycling. The electrical conductivity of PTh-based nanocomposites and their sensing capability was also reported by Ahmad [45]. Ahmad’s experimental results showed that molybdenum inclusion in PTh could enhance its electrical conductivity by 1 order of magnitude and yield a better sensitivity performance.
The metal oxide is also an excellent additive to conducting polymers due to its pseudocapacitive properties. Manganese dioxide, for instance, has excellent electrochemical behavior in neutral electrolytes, high capacity, and a wide potential window. However, metal oxides have poor electrical conductivity. Manganese dioxide prepared by hydrothermal synthesis was mixed with the nanocomposite of polyaniline-carbon nanotube prepared by oxidation polypyrrole [46]. The investigation results revealed that the electrochemical synergy between the individual material, has direct proportional effects on their nanocomposites. As shown in Fig. 9a, the reduction and oxidation process of the polyaniline-carbon nanotube-manganese dioxide nanocomposite has a higher rate at high efficiency than the other materials. Figure 9b, also provided the improvement in the specific capacity of the ternary nanocomposite.
Polyaniline-carbon nanotube-manganese dioxide nanocomposite a cyclic voltammetry b specific capacity. Reproduce with permission from Ref. [46]. ©2020, Polymer (MDPI)
A copolymer consisting of polyaniline-melamine was hybridized with cobalt tetraoxide and graphene oxide by Ahmed et al. [47]. The copolymer-reinforced cobalt /graphene oxide yielded a high specific capacity of 134.4 C g−1. In a similar study, Ahmed et al. synthesized the nanocomposite of polyaniline-melamine copolymer-reinforced tin dioxide/graphene oxide [48]. The fabrication process is provided in Fig. 10.

Reproduced with permission from Ref. [48]. ©2022, Journal of materials research, Springer
Polyaniline-melamine copolymer reinforce tin-oxide/graphene oxide nanocomposite fabrication process.
2.2 Fabrication of Polypyrrole and Polypyrrole Nanocomposites
Polypyrrole (PPy) is another conducting polymer that has been extensively researched due to its excellent properties and many application areas. Electrically conductive polymers have positive environmental impacts, low cost of production, easy hybridization methods with fillers, and good flexibility. The conjugated conductive polypyrrole has been utilized as electrodes, sensors, adsorbents of heavy metals, and biomedical applications [49,50,51,52]. Their synthesis methods, however, are of great significance to their properties, turn-ability and applications.
A recent study by Seike et al. [53], presented a solvent-free synthesis approach to fabricate PPy. The production procedures (Fig. 11) involved the mechanical mixing of pyrrole monomers with solid ferric chloride under a nitrogen atmosphere. The magnetic stirring of the resultant product was carried out at 25 °C and was allowed to polymerize for 24 h. Seike et al. [53] reported that pressed-pellet solvent-free PPy could serve as a photonic nanomaterial, which can easily convert light to heat. Photonic nanomaterials are valuable materials for cancer theranostic.

Reproduced with permission from Ref. [53]. ©2022, ACS Omega
PPy solvent-free fabrication procedures and applications.
Tin/lead solders are the most frequently used in making electronic circuit boards. Recently, the environmental hazards of tin/lead solders propelled researchers to look for better alternatives. Hence, Wen et al. [54] experimentally fabricated PPy/coated adhesives as excellent solder to replace the tin/lead solder. The synthesis of the PPy/coated adhesive was achieved by using the facile one-step chemical oxidation reaction at room temperature. Since the size of the nanoparticles are important parameters that dictate their functionality, Wen et al. controlled the diameters of the PPy/coated adhesives by using polyvinylpyrrolidone as a surfactant. The electrical resistivity of the material produced was reduced from 1.6 × 10−3Ω-cm to 9.4 × 10−5Ω-cm. In oxidative chemical polymerization, the dispersion of nanoparticles can be controlled by a suitable solvent, mixing approach, and condition of drying [55]. The PPy nanoparticles-loaded silver-epoxy, prepared by Wen et al. was reported to be flexible, and it can be used to produce electronic circuit boards.
During the fabrication of PPy and its nanocomposites, the efficiency of the dispersibility of the nanoparticles in the chosen organic solvent has some degree of advantage. Kisiel et al. [56] proposed the oxidation of pyrrole monomers by either using iron(III)-toluenesulfonate or potassium-hexacyanoferrate(III) and using poly(vinyl-alcohol) aqueous solution as dispersing medium. The aqueous poly(vinyl-alcohol) created a well-dispersed nanoparticle in solution. It can be used to eliminate the need for a polyacrylate microsphere while aiming at fabricating nanosphere-PPy containing a low percentage of the oxidizing agent; this method can be used to manufacture PPy for various applications.
The influence of polymerization method, time, and condition of PPy nanoparticles and its nanocomposite, influences their morphologies and porosities. These effects were investigated by Wilczewska et al. [57], when they composited PPy nanoparticles with graphene quantum-dot by using the oxidative chemical polymerization method. The diameter of the PPy/graphene quantum-dot nanocomposites was reported to increase as the concentration of the filler and the polymerization time increased. Hence, optimizing polymerization time and filler concentration is essential while compositing polymers with any filler type. The structural modification of PPy nanocomposites using graphene and its derivatives has been reported as a novel method to improve the electrochemical properties of PPy [58]. Graphene in polymer-nanocomposites for electrochemical electrodes acts as a charge transfer mechanizer in the electrodes. In addition, the structural modification of polymers with graphene modifies their surfaces by increasing their surface area and porosity. Therefore, it is not unlikely to expect that the nanocomposites of polymer/graphene and its derivatives will yield high-performing electrochemical electrodes.
Furthermore, conjugated conducting polypyrrole can function as a sensor due to its sensitivity stability at room temperature. Yadav et al. [59] experimentally investigated PPy-sensor performances on ammonia gas. A chemical bath deposition synthesis method was employed to produce the PPy-sensor; the sensor showed 85% sensitivity to ammonia gas at room temperature. The resistance of the polymer changed when it was exposed to the ammonia gas by either donating or accepting electrons.
The simplicity in synthesizing of PPy and PPy-based nanocomposites establishes a fascinating interest in the investigation of its properties. Khan et al. [60] reported on an overnight polymerization of PPy polymer. The polymerization was done by adding ferric chloride, methyl-orange, and pyrrole monomer in 400 ml of deionized water; the resultant solution was stirred for 24 h. Graphene nanoparticles were loaded on the PPy obtained, after filtration and drying to improve its electrocatalytic efficiency. The PPy (Fig. 12a) produced by this synthesis approach showed the spherical structure, while the dispersion of graphene nanoparticles in the PPy matrix is as shown in Fig. 12b. High degree dispersibility enhances the electrical conductivity of polymer nanocomposites. As shown in Fig. 12c, the energy conversion performances of the PPy/graphene showed the lowest performance for the pristine PPy. However, the efficiency increases when graphene creates more carrier transport, low resistance, and enhanced catalytic activities in the electrode. However, during the fabrication of PPy nanoparticles and nanocomposites, caution must be observed to avoid the agglomeration of the nanostructure since agglomeration creates an effect that hinders ionic transportation.

Reproduced with permission from Ref. [60]. ©2022, Elsevier Ltd
PPy/graphene nanocomposites a, b microstructure and b photocatalytic property.
Tumacder et al. [61] synthesized a fibrillar structure-like PPy by using the electrosynthesis method in the presence of acid-blue 25. The capacitance of the PPy electrode was reported to have increased by one order of magnitude. The electrosynthesis was carried out in a cyclic voltammetry system on a glassy-carbon electrode; the pyrrole monomer was suspended in the aqueous solution of hydrochloric acid. The globular microstructure of the PPy film, is as shown in Fig. 13a. In addition, as shown in Fig. 13b–d, the cyclic voltammetry analysis of the PPy and PPy/acid-blue-25, led to the formation of the polymers on the system electrode. Moreso, the cyclic stability efficiency of the PPy/acid blue-25 was higher than the pristine PPy. The validity and efficiency of this method are still subject to further verification since there is sparse information on this subject, in the literature.

Reproduced with permission from Ref. [61]. ©2022, Elsevier Ltd
PPy and PPy/acid-blue25 nanoparticles a microstructure b, c current density and d percentage cyclic stability.
A thermoelectric transducer converts heat energy to electrical energy by the Seebeck and Peltier effects. Polypyrrole is a polymer that can perform thermoelectric effects if an appropriate doping process tunes its physical/thermal properties. In this case, Li et al. [62] carried out an electrochemical-doping technique in order to adjust the PPy nanoparticles. The electro-polymerization of the PPy film was achieved by the mixture of isopropyl alcohol, boron-fluoride-diethyl-etherate and the pyrrole monomer at a constant voltage of 0.8 V at 0 °C, for 15 min and dried at 60 °C for 8 h. While controlling the properties of polymer materials with another material, there is always a saturating point, beyond which the polymer properties become negative. This effect was observed for the electrical conductivity of PPy film, doped with isopropyl alcohol, boron-fluoride-diethyl-etherate. As shown in Fig. 14a, as the concentration of the doping materials increased, the electrical conductivity of the polymer increased and reached its saturating point. After the saturating point, the polymer conductivity decreased to minimum. However, the Seebeck coefficient of the polymer decreased until after the saturating point, it began to increase. The thermo-electric analysis of the doped PPy film, is as shown in Fig. 14b further explained the relationship between electrical conductivity and Seebeck effect.

Reproduced with permission from Ref. [62]. ©2020, Elsevier Ltd
Doped PPy (isopropyl alcohol, boron-fluoride-diethyl-etherate) a electrical conductivity, Seebeck-coefficient, and power-factor b Thermo-electric analysis.
The continuous growth in technology, viz: telecommunication, military devices, and industrial instruments, suggests the fact that there are adequate developments in electric and magnetic wave absorbers. Nanocomposites made of conjugative polymers are suitable for the wave/electric-thermal process of absorbers. For this purpose, Maleki et al. [63] recently carried out an investigation on nanocomposite of PPy/halloysite/Fe3O4 by using the hydrothermal and in-situ polymerization synthesis methods (Fig. 15). Fe2O4 are magnetic nanoparticles with low-loss magnetic property. PPy nanocomposites are electrically conductive polymers with excellent dielectric properties and halloysite aluminosilicate-nanotube with good thermal and mechanical properties. For an electromagnetic energy storage device, their strengths and efficiencies are often determined by their permittivity and permeability. The experimentation of Maleki et al. showed that the amount of PPy content in the nanocomposite of PPy/halloysite/Fe3O4 is proportional to the conductivity and the permittivity of the nanocomposite. Moreso, the halloysite participated in the enhancement of the nanocomposite absorbance property. As wastewater absorbent, Sadeghnezhad et al. [64] experimentally produced PPy/sodium-alginate/magnetic-copper-iron-oxide nanocomposite by using the hydrothermal synthesis method. The fabricated PPy/metal oxide nanocomposite showed excellent performances in eliminating direct blue-199 dye from wastewater.

Reproduced with permission from Ref. [63]. ©2022, Elsevier Ltd
PPy/halloysite/Fe3O4 nanocomposite fabrication method.
Furthermore, Naseeb et al. [65] fabricated the nanocomposite of PPy/sodium-metavanadate for supercapacitor electrode by using an interfacial polymerization method. As shown in Fig. 16a, interfacial polymerization, is a confined chemical reaction, in which there exists a liquid/liquid or liquid/air interface. The advantage of interfacial polymerization, include: controllable fabrication of electrode nanomaterials [66]. Naseeb et al. reported that the specific energy and power densities of the PPy/metavanadate nanocomposite electrode, exhibited excellent stabilities by sustaining − 59% of capacitance after 1000-cycles. The synergy between the polymer and the metavanadate, yielded a high specific surface area for easy ionic transportation. Figure 16b shows the energy/power density curve.

Reproduced with permission from Ref. [65]. ©2022, Elsevier Ltd
a Interfacial polymerization of nanocomposites b PPy/sodium-metavanadate energy/power density.
A one-step electropolymerization synthesis method was employed by Malik et al. [67] to prepare PPy/carbon quantum dots nanocomposite electrode. A reduction in the charge transfer resistance, high electrical conductivity, and surface area, were the associated properties recorded for the electrode. The results of Malik et al. experimentation confirmed that the electropolymerization method is an excellent synthesis approach for the production of large surface area nanocomposite electrodes.
2.3 Fabrication of Graphene and Graphene-Based Nanocomposites
Graphene (Gr) is a single layered of carbon atoms; hexagonally arranged two-dimensional nanomaterial with substantive surface area, electrical conductivity and good chemical and thermal stabilities. The lateral size of graphene can range from nanometers to macroscale; therefore, graphene has a divers’ family [68]. Due to the divers’ families of graphene, there are several synthetic methods for their production. Chemical vapor deposition and mechanical and chemical exfoliation techniques, have been used to fabricate graphene and its derivatives [69, 70]. Bottom-up and top-down methods are the two categories of preparation techniques of graphene and graphene-based nanomaterials. In the bottom-up technique, the graphene nanoparticles are grown on substrates (that is, epitaxial growth of graphene on a substrate). Examples of the bottom-up synthesis techniques, are chemical vapour deposition and pyrolysis [71, 72]. The top-down method is concerned with the exfoliation of graphite; the method includes mechanical and chemical peelings, ultrasonication and liquid-phase exfoliations [73]. A proper fabrication method is required to synthesize graphene because its electrical, thermal, chemical and mechanical properties can easily, be altered by the amounts of functional elements in it.
Graphene oxide can be produced by the sonication of graphite oxide, thermal and mechanical exfoliations. However, these methods often, generate large amount of carbon dioxide and explosive thermal reduction of materials. Zaaba et al. [69] prepared graphene oxide by adding and stirring 27 ml of sulfuric acid and 3 ml of phosphoric acid for some minutes. A 225 mg of graphite oxide was added to the solution of sulfuric and phosphoric acids, under a stirring state, followed by the slow addition of 1.320 g potassium permanganate. The resultant solution was stirred for about 6 h to remove excess potassium manganate. A 0.675 ml of hydrogen peroxide was added dropwise to the stirred solution and re-stirred for about 10 min. The precipitate was collected by the centrifugation process in the presence of hydrochloric acid and deionized water. The graphene oxide obtained was dried for 24 h at 90 °C. This method was named, the modified Hummer’s method due to the reduction in toxic gases (NO2 and N2O4) achieved by replacing sodium nitrate with sulfuric and phosphoric acids. The properties of the graphene oxide obtained and its microstructure, dissolved in ethanol and acetone, are as shown in Fig. 17a–c.
Yoonessi and Gaier fabricated the nanocomposite of graphene/polycarbonate using emulsion mixing and solution blending methods [74]. The two different synthesis methods, yielded a polymer composites having two electrical conductivity at different weight fractions. However, the results of the two different methods produced a highly nanocomposite with no connectivity path for insulation (see Fig. 17e and f). Recently, graphene oxide was produced by replacing NaNO3 in the Hummer’s method, with boric acid [75]. The inhibition of carbonyl/carboxyl groups, the elimination of toxic gases, a yield increase by 10% and a reduction in the reaction time, are the recorded benefits of modifying Hummer’s synthesis method with boric acid. The fluffy sheet-like structure of the graphene oxide is shown in Fig. 18a, b. The structural identification of the graphene oxide, via the XRD, shows peaks ranging between 2θ = 9.1° and 10.1°; the thermal stability was sustainable with a total residual mass of between 46.22 and 41.43%, following a TGA investigation (Fig. 18c, d).
Hummer’s boric acid modification production of graphene oxide properties a, b microstructure c structural identification and d temperature analysis. e, f Graphene nanoplatelet high resolution transmission electron microscopy image.
Graphene nanoplatelet is an ultra-thin graphene with a thickness of less than 100 nm; it is highly electrically conductive, and it possesses excellent electrochemical performances. This type of graphene can be produced by using the facile one-step synthesis method. Chong et al. [76] employed a facile one-step synthesis method to fabricate a highly porous graphene nanoplatelet electrochemical electrode film. The facile one-step synthesis method requires low processing temperature, a simple approach and it is cost-effective, for fabricating electrochemical electrodes.
Graphene-oxide/manganese dioxide nanocomposite was fabricated by Pimklang et al. [77] by using the solution-plasma technique for electrochemical electrode applications. In this case, graphene-oxide produced by the modified Hummer’s method, was dispersed in 30 ml of distilled water via the sonication process. A measured quantity of KMnO4 was dissolved in 70 ml of distilled water and thereafter, added to the sonicated graphene-oxide. Figure 19 provides the solution plasma technique for graphene-oxide/manganese dioxide production. The solution plasma technique, requires no reducing agents; the process only lasted for − 10 min. A 218 F/g specific capacitance was recorded for the electrode produced.

Reproduced with permission from Ref. [77]. ©2022, Elsevier Ltd
Solution plasma technique production of graphene-oxide/manganese dioxide.
A hydrothermal one-step synthesis method has also been investigated to produce graphene oxide/manganese dioxide nanocomposite. The features of the nanocomposite produced by the one-step hydrothermal method, includes large surface area and high porosity, excellent electrical conductivity, and good electrochemical performances. Zhang et al. [78] reported that the nanocomposite made of graphene oxide/manganese dioxide may possibly yield a 255 F/g capacitance at 0.5 A/g current density, a retention rate of about 85% after 10,000 cycles, 33.33 Wh/kg energy density and 20,000 W/kg power densities. In addition, an essential parameter in the preparation of the sample, by using the one-pot hydrothermal technique, is the concentration of KMnO4. The potassium permanganate controls the thickness and the electrochemical performances of the resulting nanocomposite.
The energy and power densities and other performance problems of electrochemical electrodes can be ameliorated using combined materials, prepared by an adequate synthesis approach. For this reason, the exploration of the laser evaporation method for the production of reduced graphene oxide/bimetallic oxide/multi-walled carbon nanotubes was reported by Lebiere et al. [79]. The thoroughly dispersed mixture of graphene oxide, multiwalled carbon nanotubes and bimetallic oxide, was exposed to laser irradiation and further reduction of the graphene oxide was carried out by the laser pulse procedure. The benefits of the laser synthesis route, include the growth of multi-materials on a substrate without requiring temperature. Rapid charge/discharge and high storage capacity could be achieved from the nanoelectrode due to the excellent charge-storage process on the surface of the graphene and the bimetallic oxide inclusions.
Amongst the various graphene-based nanocomposites synthesis methods, a bottom-up technique, via the liquid–liquid chemical interface, is an excellent method for obtaining large-scale, large-surface area graphene nanomaterials electrodes. As shown in Fig. 20, a one-pot synthesis of graphene/polyaniline can be engaged to produce excellent electrochemical electrodes [72].

Reproduced with permission from Ref. [72]. ©2017, Elsevier Ltd
Graphene/polymer nanocomposite bottom-up one-pot synthesis method.
In summary, the bulk transfer of electrons between the sheets of typical graphene, depends on the amount of impurities, defects and functional groups that are available in the graphene sheets [80]. The production of graphene-polymer nanocomposites is an established technique that can be employed in order to produce efficient electrochemical electrodes. However, the dispersion procedure of graphene is an essential factor that must be chosen, meticulously and practiced. In the graphene and conducting polymers interface, graphene is expected to act as a support to the matrix for electroactive species. The growth of the electroactive species is proportional to the increase in the specific surface area and the enhancement in the electrochemical properties of the graphene-polymer nanocomposite. The polypyrrole and polythiophene polymers, on the other hand, improve the energy density of the resulting composite [81]. A well-dispersed graphene nanoparticle in the polypyrrole and polythiophene hybrid matrix, would yield better conductive, electrochemically stable, flexible, thermally and mechanically viable nanocomposites that can be prepared by in-situ polymerization, electropolymerization and a facile one-step synthesis method.
3 Potential Impacts of Graphene Nanoparticles on Conducting Polymers
Intrinsic conducting polymer for electrochemical energy storage has poor charge/discharge stability. This results from the loss of electrons and the formation of poly-cations, causing the anions’ intercalation to maintain electron neutrality [82]. However, repeating this process (charge/discharge) stresses the polymer more and more, resulting in poor cycling. This hampers the creation of high-power supercapacitors.[83]. Besides the poor charge/discharge characteristic of intrinsic conducting polymers, intrinsic conducting polymers have poor electrical conductivity, and slow kinetic and electron-transfer efficiency [84]. Therefore, modifying the electrochemical stability, surface area, and electrical conductivity of conductivity polymers is urgently essential.
Graphene has excellent properties, allowing the fabrication of diverse nanocomposite applications, possessing excellent electrical conductivity, stable chemical and thermal stability, and high mechanical strength at a relatively low cost. Graphene has a large surface area: a property that determines the effectiveness of ions transit between electrodes and electrolytes. The fabrication of graphene/conducting polymer nanocomposites attains an excellent fast charge/discharge process with a wide electrochemical window.
4 Conclusions and Future Research Outlook
The development of nanotechnology is currently offering relief to scientists and industrialists due to their wide range of application. Graphene, a two-dimensional material with unrivaled properties, is one of the materials capturing the interest of scientists because it offers answers in a distinct field. They are a valuable asset in energy and environmental industries due to their exceptional electrochemical properties. Graphene and graphene-related materials, despite their outstanding properties have energy band gap which limit their functional and economic applications [7]. Interestingly, these drawbacks can be overcome by hybridizing conjugated polymers with graphene. Polypyrrole and polythiophene are two notable conjugated polymers, with high electrical conductivity that can be used in energy storage systems, solar cells, sensors and other applications [8].
To achieve this, synthesis techniques play a significant role, which is why this study summarized the recent studies on the hybrid formation of polypyrrole/polythiophene-loaded graphene for various applications, including its energy storage capabilities. The study analyzed various synthesis methods of producing polypyrrole, polythiophene, graphene, and their composites. The influence of the polymerization method, time/duration of polymerization, and the conditions for conductive polymer nanoparticles and their nanocomposites dictate their morphologies and porosities. As a result, when compositing polymers with any filler type, optimizing the polymerization time and filler concentration is critical. More so, a high degree of dispersibility enhances the electrical conductivity of polymer nanocomposites. Electropolymerization, in-situ polymerization, and facile one-step synthesis methods can reduce charge transfer resistance, high electrical conductivity, and nanoelectrode’s surface area to volume ratio.
Furthermore, by considering the exceptional electrochemical performances of polypyrrole, polythiophene and graphene, this study proposes the fabrication of their composite by using an appropriate synthesis method fo energy storage applications. More research on fabrication of these composite for energy storage electode taking into account optimization of polymerization time/duration and filler concentration is suggested. The use of computational techniques and simulation are other viable techniques to examine the properties of composite materials. These techniques have tendency to reveal additional valiable technical information that experimentation studies may not discover. Future research studies in this area, are this advised. A composite material emanating from the hybrid configuration of graphene and conducting polymers is envisaged to offer potential with excellent properties. This composite material has a tendecncy to handle current energy storage system crisis and proffer solutions to the the next energy generation and environmental challenges.
References
S.P. Nathaniel, K. Yalçiner, F.V. Bekun, Assessing the environmental sustainability corridor: Linking natural resources, renewable energy, human capital, and ecological footprint in BRICS. Resour. Policy 70, 101924 (2021)
M.T. Hussain, N.B. Sulaiman, M.S. Hussain, M. Jabir, Optimal management strategies to solve issues of grid having electric vehicles (EV): A review. J. Energy Storage 33, 102114 (2021)
A. Manthiram, A.V. Murugan, A. Sarkar, T. Muraliganth, Nanostructured electrode materials for electrochemical energy storage and conversion. Energy Environ. Sci. 1(6), 621–638 (2008)
Y. Ma, H. Chang, M. Zhang, Y. Chen, Graphene-based materials for lithium-ion hybrid supercapacitors. Adv. Mater. 27(36), 5296–5308 (2015)
R. Fang, K. Chen, L. Yin, Z. Sun, F. Li, H.M. Cheng, The regulating role of carbon nanotubes and graphene in lithium-ion and lithium–sulfur batteries. Adv. Mater. 31(9), 1800863 (2019)
A. Chaudhuri, A. Chaudhuri, A. Joydhar, Graphene nanocomposites and applications in electrochemical energy storage materials. Mater. Today: Proc. 64, 1569–1581 (2022)
K.Y. Rhee, Electronic and thermal properties of graphene. Multidisc Digital Publ Inst 10, 926 (2020)
H. Lu, X. Li, Q. Lei, Conjugated conductive polymer materials and its applications: A mini-review. Front. Chem. 9, 728 (2021)
A.T. Dideikin, A.Y. Vul’, Graphene oxide and derivatives: The place in graphene family. Front. Phys. 6, 149 (2019)
Y. Li, M. Zhou, Y. Wang, Q. Pan, Q. Gong, Z. Xia et al., Remarkably enhanced performances of novel polythiophene-grafting-graphene oxide composite via long alkoxy linkage for supercapacitor application. Carbon 147, 519–531 (2019)
X. Ye, L. Yang, Z. Tian, P. Zhou, S. Wang, H. Sun et al., Polypyrrole-coated conjugated microporous polymers/expanded graphene carbon aerogels based phase change materials composites for efficient energy conversion and storage. Sol. Energy Mater. Sol. Cells 245, 111873 (2022)
K. Kong, W. Xue, W. Zhu, W. Ye, Z. Zhang, R. Zhao et al., The fabrication of bowl-shaped polypyrrole/graphene nanostructural electrodes and its application in all-solid-state supercapacitor devices. J. Power Sources 470, 228452 (2020)
Z. Zhao, H. Wang, H. Huang, L. Li, X. Yu, Graphene oxide/polypyrrole/polyaniline composite hydrogel synthesized by vapor-liquid interfacial method for supercapacitors. Colloids Surf. A 626, 127125 (2021)
R. Xie, J. Fan, K. Fang, W. Chen, Y. Song, Y. Pan et al., Hierarchical Bi2MoO6 microsphere photocatalysts modified with polypyrrole conjugated polymer for efficient decontamination of organic pollutants. Chemosphere 286, 131541 (2022)
K. Pal, A. Si, G.S. El-Sayyad, M.A. Elkodous, R. Kumar, A.I. El-Batal et al., Cutting edge development on graphene derivatives modified by liquid crystal and CdS/TiO2 hybrid matrix: optoelectronics and biotechnological aspects. Crit. Rev. Solid State Mater. Sci. 46(5), 385–449 (2021)
X. Wang, J. Xiao, W. Tang, Hydroquinone versus pyrocatechol pendants twisted conjugated polymer cathodes for high-performance and robust aqueous zinc-ion batteries. Adv. Func. Mater. 32(4), 2108225 (2022)
S. Asiya, G.Z. Kyzas, K. Pal, J.F.G. de Souza, Graphene functionalized hybrid nanomaterials for industrial-scale applications: A systematic review. J. Mol. Struct. 1239, 130518 (2021)
K. Pal, N. Asthana, A.A. Aljabali, S.K. Bhardwaj, S. Kralj, A. Penkova et al., A critical review on multifunctional smart materials ‘nanographene’emerging avenue: Nano-imaging and biosensor applications. Crit. Rev. Solid State Mater. Sci. 47(5), 691–707 (2022)
P. Vahdatiyekta, M. Zniber, J. Bobacka, T.-P. Huynh, A review on conjugated polymer-based electronic tongues. Analy Chim Acta 1221, 340114 (2022)
S. Bagyalakshmi, A. Sivakami, K. Pal, R. Sarankumar, C. Mahendran, Manufacturing of electrochemical sensors via carbon nanomaterials novel applications: a systematic review. J. Nanopart. Res. 24(10), 201 (2022)
S. Kang, T.W. Yoon, G.-Y. Kim, B. Kang, Review of conjugated polymer nanoparticles: From formulation to applications. ACS Appl. Nano Mater. 5(12), 17436–17460 (2022)
S. Soren, S. Chakroborty, K. Pal, Enhanced in tunning of photochemical and electrochemical responses of inorganic metal oxide nanoparticles via rGO frameworks (MO/rGO): A comprehensive review. Mater. Sci. Eng., B 278, 115632 (2022)
E. Kramer, J. Podurgiel, M. Wei, Control of hydroxyapatite nanoparticle morphology using wet synthesis techniques: Reactant addition rate effects. Mater. Lett. 131, 145–147 (2014)
J.K. Patra, K.-H. Baek, Green nanobiotechnology: Factors affecting synthesis and characterization techniques. J. Nanomater. 2014, 1–12 (2014)
Y. Lu, S. Wang, C. Xiong, G.-H. Hu, Synthesis and characterization of a liquid-like polythiophene and its potential applications. Synth. Met. 270, 116603 (2020)
S.R.P. Gnanakan, M. Rajasekhar, A. Subramania, Synthesis of polythiophene nanoparticles by surfactant-assisted dilute polymerization method for high performance redox supercapacitors. Int. J. Electrochem. Sci 4(9), 1289–1301 (2009)
P. Pascariu, D. Vernardou, M.P. Suchea, A. Airinei, L. Ursu, S. Bucur et al., Tuning electrical properties of polythiophene/nickel nanocomposites via fabrication. Mater. Des. 182, 108027 (2019)
Q.-T. Vu, M. Pavlik, N. Hebestreit, U. Rammelt, W. Plieth, J. Pfleger, Nanocomposites based on titanium dioxide and polythiophene: Structure and properties. React. Funct. Polym. 65(1–2), 69–77 (2005)
G. Shi, J. Xu, M. Fu, Raman spectroscopic and electrochemical studies on the doping level changes of polythiophene films during their electrochemical growth processes. J. Phys. Chem. B 106(2), 288–292 (2002)
P. Sundriyal, M. Sahu, O. Prakash, S. Bhattacharya, Recent advancement in the fabrication of energy storage devices for miniaturized electronics, in Nano-energetic materials. (Springer, Singapore, 2019), pp.215–240
A.M. Khattak, H. Sin, Z.A. Ghazi, X. He, B. Liang, N.A. Khan et al., Controllable fabrication of redox-active conjugated microporous polymers on reduced graphene oxide for high performance faradaic energy storage. J. Mater. Chem. A 6(39), 18827–18832 (2018)
S. Jin, M. Ji, G. Xue, Electrochemical fabrication of a novel conducting polythiophene film junction. Appl. Phys. A 63(4), 397–398 (1996)
H.H. Al-Refai, A.A. Ganash, M.A. Hussein, Polythiophene and its derivatives–based nanocomposites in electrochemical sensing: A mini review. Mater. Today Commun. 26, 101935 (2021)
A.K. Thakur, M. Majumder, R.B. Choudhary, S.N. Pimpalkar, Supercapacitor based on electropolymerized polythiophene and multiwalled carbon nanotubes composites. In IOP Conf. Ser.: Mater. Sci. Eng. 149(1), 012166 (2016)
H. Vijeth, M. Niranjana, L. Yesappa, S. Ashokkumar, H. Devendrappa, Polythiophene nanocomposites as high performance electrode material for supercapacitor application. AIP Conf. Proc. 1942(1), 140017 (2018)
D. Kong, H. Fan, X. Ding, D. Wang, S. Tian, H. Hu et al., β-hydrogen of polythiophene induced aluminum ion storage for high-performance Al-polythiophene batteries. ACS Appl. Mater. Interfaces. 12(41), 46065–46072 (2020)
X.-Z. Guo, Y.-F. Kang, T.-L. Yang, S.-R. Wang, Low-temperature NO2 sensors based on polythiophene/WO3 organic-inorganic hybrids. Trans. Nonferrous Metals Soc. China 22(2), 380–385 (2012)
Q. Lu, Y. Zhou, Synthesis of mesoporous polythiophene/MnO2 nanocomposite and its enhanced pseudocapacitive properties. J. Power Sources 196(8), 4088–4094 (2011)
A. Haghgir, S.H. Hosseini, M. Tanzifi, M.T. Yaraki, B. Bayati, T. Saemian et al., Synthesis of polythiophene/zeolite/iron nanocomposite for adsorptive remediation of azo dye: Optimized by Taguchi method. Chem. Eng. Res. Design 183, 525–537 (2022)
T. Hellmann, C.S. Inagaki, M.F. das Neves, M.M. Oliveira, L.S. Roman, A.J. Zarbin et al., Preparation and characterization of polythiophene/gold nanoparticles/carbon nanotubes nanocomposites thin films: Spectroscopy and morphology. Mater. Today Commun. 33, 104314 (2022)
K. Amruth, K. Abhirami, S. Sankar, M. Ramesan, Synthesis, characterization, dielectric properties and gas sensing application of polythiophene/chitosan nanocomposites. Inorg. Chem. Commun. 136, 109184 (2022)
H. Noreen, J. Iqbal, W. Hassan, G. Rahman, M. Yaseen, A.U. Rahman, Synthesis of graphene nanoplatelets/polythiophene nanocomposites with enhanced photocatalytic degradation of bromophenol blue and antibacterial properties. Mater. Res. Bull. 142, 111435 (2021)
M.-S. Tsai, P.-G. Su, C.-J. Lu, Fabrication of a highly sensitive flexible humidity sensor based on Pt/polythiophene/reduced graphene oxide ternary nanocomposite films using a simple one-pot method. Sens. Actuators, B Chem. 324, 128728 (2020)
N. Ali, M. Bilal, A. Khan, F. Ali, H. Khan, H.A. Khan et al., Fabrication strategies for functionalized nanomaterials, in Nanomaterials synthesis, characterization, hazards and safety. (Elsevier, 2021), pp.55–95
A. Husain, Electrical conductivity based ammonia, methanol and acetone vapour sensing studies on newly synthesized polythiophene/molybdenum oxide nanocomposite. J Sci Adv Materi Devices 6(4), 528–537 (2021)
J. Iqbal, M.O. Ansari, A. Numan, S. Wageh, A. Al-Ghamdi, M.G. Alam et al., Hydrothermally assisted synthesis of porous polyaniline@ carbon nanotubes–manganese dioxide ternary composite for potential application in supercapattery. Polymers 12(12), 2918 (2020)
I. Ahmed, S. Wageh, W. Rehman, J. Iqbal, S. Mir, A. Al-Ghamdi et al., Evaluation of the synergistic effect of graphene oxide sheets and co3o4 wrapped with vertically aligned arrays of poly (aniline-co-melamine) nanofibers for energy storage applications. Polymers 14(13), 2685 (2022)
I. Ahmed, W. Rehman, S. Mir, H. Somaily, M. Khalid, A. Numan, Graphene oxide sheets wrapped with poly (aniline-co-melamine) nanofibers furnished with SnO2 nanoparticles for electrochemical energy storage. J. Mater. Res. 37(21), 3505–3521 (2022)
O. Folorunso, Y. Hamam, R. Sadiku, S.S. Ray, N. Kumar, Investigation and modeling of the electrical conductivity of graphene nanoplatelets-loaded doped-polypyrrole. Polymers 13(7), 1034 (2021)
R. Ravichandran, S. Sundarrajan, J.R. Venugopal, S. Mukherjee, S. Ramakrishna, Applications of conducting polymers and their issues in biomedical engineering. J R Soc Interface 7(5), S559–S579 (2010)
S.J. Park, C.S. Park, H. Yoon, Chemo-electrical gas sensors based on conducting polymer hybrids. Polymers 9(5), 155 (2017)
M. Lo, N. Ktari, D. Gningue-Sall, A. Madani, S.E. Aaron, J.-J. Aaron et al., Polypyrrole: A reactive and functional conductive polymer for the selective electrochemical detection of heavy metals in water. Emergent Mater 3(6), 815–839 (2020)
M. Seike, M. Uda, T. Suzuki, H. Minami, S. Higashimoto, T. Hirai et al., Synthesis of polypyrrole and its derivatives as a liquid marble stabilizer via a solvent-free chemical oxidative polymerization protocol. ACS Omega 7(15), 13010–13021 (2022)
J. Wen, Y. Tian, Z. Mei, W. Wu, Y. Tian, Synthesis of polypyrrole nanoparticles and their applications in electrically conductive adhesives for improving conductivity. RSC Adv. 7(84), 53219–53225 (2017)
H.-K. Chan, P.C.L. Kwok, Production methods for nanodrug particles using the bottom-up approach. Adv. Drug Deliv. Rev. 63(6), 406–416 (2011)
A. Kisiel, D. Korol, A. Michalska, K. Maksymiuk, Polypyrrole nanoparticles of high electroactivity: Simple synthesis methods and studies on electrochemical properties. Electrochim. Acta 390, 138787 (2021)
W. Patrycja, B. Joanna, M.B. Diana, W.-Ż Monika, G. Jakub, B. Anna et al., Enhancement of polypyrrole electrochemical performance with graphene quantum dots in polypyrrole nanoparticle/graphene quantum dot composites. J Electroanaly Chem 923, 116767 (2022)
O. Folorunso, N. Kumar, Y. Hamam, R. Sadiku, S.S. Ray, Facile solvent/drying fabrication of PVA/PPy/rGO: A novel nanocomposite for energy storage applications. Results Mater. 15, 100295 (2022)
A. Yadav, S. Kulkarni, C. Lokhande, Synthesis and characterization of polypyrrole thin film by MW-CBD method for NH3 gas sensor. Polym. Bull. 75(10), 4547–4553 (2018)
I.M. Khan, R. Nazar, U. Mehmood, Development of polypyrrole/graphene (PPY/graphene) based electrocatalyst for platinum free dye-sensitized solar cells (DSSCs). Mater. Lett. 320, 132331 (2022)
D. Von Tumacder, Z. Morávková, P. Bober, Enhanced electrochemical performance of electrosynthesized fibrillar polypyrrole film. Mater. Lett. 308, 131295 (2022)
M. Li, C. Luo, J. Zhang, J. Yang, J. Xu, W. Yao et al., Electrochemical doping tuning of flexible polypyrrole film with enhanced thermoelectric performance. Surfaces Interfaces 21, 100759 (2020)
S.T. Maleki, M. Babamoradi, M. Rouhi, A. Maleki, Z. Hajizadeh, Facile hydrothermal synthesis and microwave absorption of halloysite/polypyrrole/Fe3O4. Synth. Met. 290, 117142 (2022)
M. Sadeghnezhad, M. Ghorbani, M. Nikzad, Synthesis of magnetic polypyrrole modified sodium alginate nanocomposite with excellent antibacterial properties and optimization of dye removal performance using RSM. Ind. Crops Prod. 186, 115192 (2022)
I. Naseeb, H.A. Almashhadani, R.R. Macadangdang Jr., S. Ullah, M.F. Khan, M. Kamran et al., Interfacial polymerization synthesis of polypyrrole and sodium metavanadate (PPy/NaVO3) composite as an excellent performance electrode for supercapacitors. Results Chem. 4, 100446 (2022)
F. Zhang, J.B. Fan, S. Wang, Interfacial polymerization: From chemistry to functional materials. Angew. Chem. Int. Ed. 59(49), 21840–21856 (2020)
R. Malik, S. Lata, U. Soni, P. Rani, R.S. Malik, Carbon quantum dots intercalated in polypyrrole (PPy) thin electrodes for accelerated energy storage. Electrochim. Acta 364, 137281 (2020)
A. Bianco, H.-M. Cheng, T. Enoki, Y. Gogotsi, R.H. Hurt, N. Koratkar et al., All in the graphene family–A recommended nomenclature for two-dimensional carbon materials. Carbon 65, 1–6 (2013)
N. Zaaba, K. Foo, U. Hashim, S. Tan, W.-W. Liu, C. Voon, Synthesis of graphene oxide using modified hummers method: Solvent influence. Procedia Eng. 184, 469–477 (2017)
O. Folorunso, Y. Hamam, R. Sadiku, S.S. Ray, G.J. Adekoya, Synthesis methods of borophene, graphene-loaded polypyrrole nanocomposites and their benefits for energy storage applications: A brief overview. FlatChem 26, 100211 (2021)
C. Neumann, D. Kaiser, M.J. Mohn, M. Füser, N.-E. Weber, O. Reimer et al., Bottom-up synthesis of graphene monolayers with tunable crystallinity and porosity. ACS Nano 13(6), 7310–7322 (2019)
V.H. Souza, M.M. Oliveira, A.J. Zarbin, Bottom-up synthesis of graphene/polyaniline nanocomposites for flexible and transparent energy storage devices. J. Power Sources 348, 87–93 (2017)
N. Kumar, R. Salehiyan, V. Chauke, O.J. Botlhoko, K. Setshedi, M. Scriba et al., Top-down synthesis of graphene: A comprehensive review. FlatChem 27, 100224 (2021)
M. Yoonessi, J.R. Gaier, Highly conductive multifunctional graphene polycarbonate nanocomposites. ACS Nano 4(12), 7211–7220 (2010)
Q. Zhang, Y. Yang, H. Fan, L. Feng, G. Wen, L.-C. Qin, Synthesis of graphene oxide using boric acid in hummers method. Colloids Surf. A 652, 129802 (2022)
W.G. Chong, Z.I. Ng, S.L. Yap, C.Y. Foo, H. Jiang, H. Guo et al., Facile fabrication of freestanding graphene nanoplatelets composite electrodes for multi battery storage. Mater. Today Commun. 31, 103782 (2022)
T. Pimklang, A. Watthanaphanit, P. Pakawatpanurut, Novel green synthesis of graphene oxide-manganese dioxide using solution plasma process for energy storage. Chem. Eng. J. 442, 136244 (2022)
Q. Zhang, X. Wu, Q. Zhang, F. Yang, H. Dong, J. Sui et al., One-step hydrothermal synthesis of MnO2/graphene composite for electrochemical energy storage. J. Electroanal. Chem. 837, 108–115 (2019)
P.G. Lebière, A.P. del Pino, C. Logofatu, E. György, Laser synthesis of NixZnyO/reduced graphene oxide/carbon nanotube electrodes for energy storage applications. Appl. Surf. Sci. 563, 150234 (2021)
A. Ambrosi, C.K. Chua, A. Bonanni, M. Pumera, Electrochemistry of graphene and related materials. Chem. Rev. 114(14), 7150–7188 (2014)
R. Raccichini, A. Varzi, S. Passerini, B. Scrosati, The role of graphene for electrochemical energy storage. Nat. Mater. 14(3), 271–279 (2015)
J.S. Shayeh, A. Ehsani, M. Ganjali, P. Norouzi, B. Jaleh, Conductive polymer/reduced graphene oxide/Au nano particles as efficient composite materials in electrochemical supercapacitors. Appl. Surf. Sci. 353, 594–599 (2015)
F. Alvi, M.K. Ram, P.A. Basnayaka, E. Stefanakos, Y. Goswami, A. Kumar, Graphene–polyethylenedioxythiophene conducting polymer nanocomposite based supercapacitor. Electrochim. Acta 56(25), 9406–9412 (2011)
J. Wang, J. Wang, Z. Kong, K. Lv, C. Teng, Y. Zhu, Conducting-polymer-based materials for electrochemical energy conversion and storage. Adv. Mater. 29(45), 1703044 (2017)
S. Singh, M.R. Hasan, P. Sharma, J. Narang, Graphene nanomaterials: The wondering material from synthesis to applications. Sensors Int. 3, 100190 (2022)
Acknowledgements
The authors would like to thank the Tshwane University of Technology, Pretoria, for their financial support.
Funding
Open access funding provided by Tshwane University of Technology. The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
OSA: Conceptualization, methodology, Writing- original draft and review. RS: Conceptualization, and Writing- editing and review. YH Writing- review and editing.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interest or personal relationship that could have influenced the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Adedoja, O.S., Sadiku, E.R. & Hamam, Y. Prospects of Hybrid Conjugated Polymers Loaded Graphene in Electrochemical Energy Storage Applications. J Inorg Organomet Polym 33, 3915–3934 (2023). https://doi.org/10.1007/s10904-023-02664-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-023-02664-2