Abstract
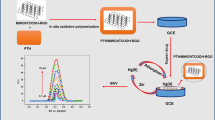
In this study, we describe an electrochemical method for detecting Cd(II) ions, utilizing a glassy carbon electrode (GCE) modified with polythiophene (PTH)/multi-walled carbon nanotubes with grapheme (MECNTs-G), chitosan (CS), and CuO. The PTH/MWCNTs-G/CS/CuO was prepared via an in situ oxidative polymerization route. XRD and FT-TR analysis confirmed that the nanocomposites were successfully prepared. TEM, SEM, EDX, TGA, and DTG were utilized to study the morphological and thermal properties of the newly synthesized materials. The electrochemical detection of Cd(II) was achieved by CV and SWV. The cadmium ions exhibited a linear relationship at concentrations of 30–0.01 µM with the PTH/MWCNTs-G/CS/CuO/GCE. The limit of detection and limit of quantification were found to be 0.014 and 0.049 µM, respectively. The stability, selectivity, and reproducibility of the modified electrode were also studied. Good recovery was achieved for the practical application of PTH/MWCNTs-G/CS/CuO/GCE in a tap-water sample.
Graphic Abstract
















Similar content being viewed by others
References
P. Sengupta, K. Pramanik, P. Sarkar, Simultaneous detection of trace Pb (II), Cd (II) and Hg (II) by anodic stripping analyses with glassy carbon electrode modified by solid phase synthesized iron-aluminate nano particles. Sens. Actuators B Chem. 329, 129052 (2020)
M.-L. Lin, S.-J. Jiang, Determination of As, Cd, Hg and Pb in herbs using slurry sampling electrothermal vaporisation inductively coupled plasma mass spectrometry. Food Chem. 141(3), 2158–2162 (2013)
M.S. Jiménez, M.T. Gómez, J.R. Castillo, Multi-element analysis of compost by laser ablation-inductively coupled plasma mass spectrometry. Talanta 72(3), 1141–1148 (2007)
Q. Zhou, M. Lei, Y. Liu, Y. Wu, Y. Yuan, Simultaneous determination of cadmium, lead and mercury ions at trace level by magnetic solid phase extraction with Fe@ Ag@ Dimercaptobenzene coupled to high performance liquid chromatography. Talanta 175, 194–199 (2017)
M. Thirumalai, S.N. Kumar, D. Prabhakaran, N. Sivaraman, M.A. Maheswari, Dynamically modified C18 silica monolithic column for the rapid determinations of lead, cadmium and mercury ions by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 1569, 62–69 (2018)
S. Li et al., Electrochemical microfluidics techniques for heavy metal ion detection. Analyst 143(18), 4230–4246 (2018)
X. Zheng et al., Highly sensitive determination of lead (ii) and cadmium (ii) by a large surface area mesoporous alumina modified carbon paste electrode. Rsc Adv. 8(14), 7883–7891 (2018)
C. Göde, M.L. Yola, A. Yılmaz, N. Atar, S. Wang, A novel electrochemical sensor based on calixarene functionalized reduced graphene oxide: application to simultaneous determination of Fe (III), Cd (II) and Pb (II) ions. J. Colloid Interface Sci. 508, 525–531 (2017)
W. Wu et al., Simultaneous voltammetric determination of cadmium (II), lead (II), mercury (II), zinc (II), and copper (II) using a glassy carbon electrode modified with magnetite (Fe 3 O 4) nanoparticles and fluorinated multiwalled carbon nanotubes. Microchim. Acta 186(2), 1–10 (2019)
A. Moutcine et al., Preparation, characterization and simultaneous electrochemical detection toward Cd (II) and Hg (II) of a phosphate/zinc oxide modified carbon paste electrode. Inorg. Chem. Commun. 116, 107911 (2020)
H. Dai, N. Wang, D. Wang, H. Ma, M. Lin, An electrochemical sensor based on phytic acid functionalized polypyrrole/graphene oxide nanocomposites for simultaneous determination of Cd (II) and Pb (II). Chem. Eng. J. 299, 150–155 (2016)
Y. Liu, J. Tang, X. Chen, J.H. Xin, Decoration of carbon nanotubes with chitosan. Carbon N. Y. 43(15), 3178–3180 (2005)
C.K.S. Pillai, W. Paul, C.P. Sharma, Chitin and chitosan polymers: chemistry, solubility and fiber formation. Prog. Polym. Sci. 34(7), 641–678 (2009)
I. Younes, M. Rinaudo, Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 13(3), 1133–1174 (2015)
S.D. Guna Vathana, J. Wilson, R. Prashanthi, A.C. Peter, CuO nanoflakes anchored Polythiophene nanocomposite: voltammetric detection of L-Tryptophan. Inorg. Chem. Commun. 124, 108398 (2020)
S.A. Alqarni, M.A. Hussein, A.A. Ganash, Highly sensitive and selective electrochemical determination of sunset yellow in food products based on AuNPs/PANI-co-PoAN-co-PoT/GO/Au electrode. ChemistrySelect 3(46), 13167–13177 (2018). https://doi.org/10.1002/slct.201802528
M.A. Hussein, A.A. Ganash, S.A. Alqarni, Electrochemical sensor-based gold nanoparticle/poly(aniline-co-o-toluidine)/ graphene oxide nanocomposite modified electrode for hexavalent chromium detection: a real test sample. Polym. Technol. Mater. 58(13), 1423–1436 (2019). https://doi.org/10.1080/25740881.2018.1563121
D.F. Katowah et al., Selective Hg2+ sensor performance based various carbon-nanofillers into CuO-PMMA nanocomposites. Polym. Adv. Technol. 31(9), 1946–1962 (2020)
D.F. Katowah, M.A. Hussein, M.M. Rahman, Q.A. Alsulami, M.M. Alam, A.M. Asiri, Fabrication of hybrid PVA-PVC/SnZnOx/SWCNTs nanocomposites as Sn2+ ionic probe for environmental safety. Polym. Technol. Mater. 59(6), 642–657 (2020)
D.F. Katowah et al., Selective fabrication of an electrochemical sensor for Pb2+ based on Poly (pyrrole-co-o–toluidine)/CoFe2O4 Nanocomposites. ChemistrySelect 4(35), 10609–10619 (2019)
D.F. Katowah et al., The performance of various SWCNT loading into CuO–PMMA nanocomposites towards the detection of Mn 2+ ions. J. Inorg. Organomet. Polym. Mater. 30, 5024–5041 (2020)
D.F. Katowah et al., Designed network of ternary core-shell PPCOT/NiFe2O4/C-SWCNTs nanocomposites. A Selective Fe3+ ionic sensor. J. Alloys Compd 834, 155020 (2020)
K. Rovina, S. Siddiquee, S. Md Shaarani, An electrochemical sensor for the determination of tartrazine based on CHIT/GO/MWCNTs/AuNPs composite film modified glassy carbon electrode. Drug Chem. Toxicol 44, 447 (2019)
A. Husain, S. Ahmad, F. Mohammad, Synthesis, characterisation and ethanol sensing application of polythiophene/graphene nanocomposite. Mater. Chem. Phys. (2020). https://doi.org/10.1016/j.matchemphys.2019.122324
A. Husain, S. Ahmad, F. Mohammad, Electrical conductivity and alcohol sensing studies on polythiophene/tin oxide nanocomposites. J. Sci. Adv. Mater. Devices 5(1), 84–94 (2020). https://doi.org/10.1016/j.jsamd.2020.01.002
D.F. Katowah et al., Ternary nanocomposite based poly(pyrrole-co-O-toluidine), cobalt ferrite and decorated chitosan as a selective Co2+ cationic sensor. Compos. Part B-Eng. (2019). https://doi.org/10.1016/j.compositesb.2019.107175
B. Senthilkumar, P. Thenamirtham, R.K. Selvan, Structural and electrochemical properties of polythiophene. Appl. Surf. Sci. 257(21), 9063–9067 (2011)
J. Mårdalen, E.J. Samuelsen, O.R. Gautun, P.H. Carlsen, X-ray scattering from oriented poly (3-alkylthiophenes). Synth. Met. 48(3), 363–380 (1992)
Y. Li, G. Vamvounis, S. Holdcroft, Tuning optical properties and enhancing solid-state emission of poly (thiophene) s by molecular control: a postfunctionalization approach. Macromolecules 35(18), 6900–6906 (2002)
T.S. Swathy, M.J. Antony, Tangled silver nanoparticles embedded polythiophene-functionalized multiwalled carbon nanotube nanocomposites with remarkable electrical and thermal properties”. Polymer (Guildf) 189, 122171 (2020)
M.E.I. Badawy, T.M.R. Lotfy, S.M.S. Shawir, Preparation and antibacterial activity of chitosan-silver nanoparticles for application in preservation of minced meat. Bull. Natl. Res. Cent. 43(1), 1–14 (2019)
D. Thanasamy, D. Jesuraj, V. Avadhanam, A novel route to synthesis polythiophene with great yield and high electrical conductivity without post doping process. Polymer (Guildf) 175, 32–40 (2019)
F.A. Harraz, M. Faisal, M. Jalalah, A.A. Almadiy, S.A. Al-Sayari, M.S. Al-Assiri, Conducting polythiophene/alpha-Fe2O3 nanocomposite for efficient methanol electrochemical sensor. Appl. Surf. Sci. (2020). https://doi.org/10.1016/j.apsusc.2019.145226
Y. Xie et al., Preparation and electromagnetic properties of chitosan-decorated ferrite-filled multi-walled carbon nanotubes/polythiophene composites. Compos. Sci. Technol. 99, 141–146 (2014)
S.D. GunaVathana, P. Thivya, J. Wilson, A.C. Peter, Sensitive voltammetric sensor based on silver dendrites decorated polythiophene nanocomposite: Selective determination of L-Tryptophan. J. Mol. Struct. (2020). https://doi.org/10.1016/j.molstruc.2019.127649
F. Avilés, J.V. Cauich-Rodríguez, L. Moo-Tah, A. May-Pat, R. Vargas-Coronado, Evaluation of mild acid oxidation treatments for MWCNT functionalization. Carbon N. Y. 47(13), 2970–2975 (2009)
C.L. Poh, M. Mariatti, A.F.M. Noor, O. Sidek, T.P. Chuah, S.C. Chow, Dielectric properties of surface treated multi-walled carbon nanotube/epoxy thin film composites. Compos. Part B Eng. 85, 50–58 (2016)
M. Senel et al., Enhanced electrochemical sensing performance by in situ electrocopolymerization of pyrrole and thiophene-grafted chitosan. Int. J. Biol. Macromol. 143, 582–593 (2020). https://doi.org/10.1016/j.ijbiomac.2019.12.013
H. Vijeth, S.P. Ashokkumar, L. Yesappa, M. Vandana, H. Devendrappa, Camphor sulfonic acid surfactant assisted polythiophene nanocomposite for efficient electrochemical hydrazine sensor. Mater. Res. Express (2019). https://doi.org/10.1088/2053-1591/ab5ef5
T. Ramachandran, V.V. Dhayabaran, Utilization of a MnO2/polythiophene/rGO nanocomposite modified glassy carbon electrode as an electrochemical sensor for methyl parathion. J. Mater. Sci. Electron. 30(13), 12315–12327 (2019). https://doi.org/10.1007/s10854-019-01590-9
J. Zhao et al., Preparation and characterization of an electromagnetic material: the graphene nanosheet/polythiophene composite. Synth. Met. 181, 110–116 (2013)
B.X. Valderrama-García, E. Rodríguez-Alba, E.G. Morales-Espinoza, K. Moineau Chane-Ching, E. Rivera, Synthesis and characterization of novel polythiophenes containing pyrene chromophores: thermal, optical and electrochemical properties. Molecules 21(2), 172 (2016)
A.K. Thakur, M. Majumder, R.B. Choudhary, S.N. Pimpalkar, Supercapacitor based on electropolymerized polythiophene and multiwalled carbon nanotubes composites. IOP Conf. Ser.: Mater. Sci. Eng. 149(1), 12166 (2016)
S. Khanmohammadi et al., Polythiophene/TiO2 and polythiophene/ZrO2 nanocomposites: physical and antimicrobial properties against common infections. Biointerface Res. Appl. Chem. 8(4), 3457–3462 (2018)
B. Singh, R.-A. Doong, D.S. Chauhan, A.K. Dubey, Synthesis and characterization of Fe3O4/Polythiophene hybrid nanocomposites for electroanalytical application”. Mater. Chem. Phys. 205, 462–469 (2018). https://doi.org/10.1016/j.matchemphys.2017.11.040
M.A. Hussein, B.M. Abu-Zied, A.M. Asiri, The role of mixed graphene/carbon nanotubes on the coating performance of G/CNTs/epoxy resin nanocomposites. Int. J. Electrochem. Sci 11, 7644–7659 (2016)
D.F. Katowah, G.I. Mohammed, D.A. Al-Eryani, O.I. Osman, T.R. Sobahi, M.A. Hussein, Fabrication of conductive cross-linked polyaniline/G-MWCNTS core-shell nanocomposite: a selective sensor for trace determination of chlorophenol in water samples. Polym. Adv. Technol. 31(11), 2615–2631 (2020)
S. Parvez et al., Preparation and characterization of artificial skin using chitosan and gelatin composites for potential biomedical application. Polym. Bull. 69(6), 715–731 (2012)
G. Ma, D. Yang, J.F. Kennedy, J. Nie, Synthesize and characterization of organic-soluble acylated chitosan. Carbohydr. Polym. 75(3), 390–394 (2009)
A.H. Gedam, R.S. Dongre, Adsorption characterization of Pb (II) ions onto iodate doped chitosan composite: equilibrium and kinetic studies. RSC Adv. 5(67), 54188–54201 (2015)
A. Bard, Electrochemical Merhods. Fundamentals and Applications, vol. 290 (Wiley, New York, 1980)
R. Madhuvilakku, S. Alagar, R. Mariappan, S. Piraman, Glassy carbon electrodes modified with reduced graphene oxide-MoS2-poly (3, 4-ethylene dioxythiophene) nanocomposites for the non-enzymatic detection of nitrite in water and milk. Anal. Chim. Acta 1093, 93–105 (2020). https://doi.org/10.1016/j.aca.2019.09.043
R. Pauliukaite, M.E. Ghica, O. Fatibello-Filho, C.M.A. Brett, Electrochemical impedance studies of chitosan-modified electrodes for application in electrochemical sensors and biosensors. Electrochim. Acta 55(21), 6239–6247 (2010). https://doi.org/10.1016/j.electacta.2009.09.055
S. Sakthinathan et al., Platinum incorporated mordenite zeolite modified glassy carbon electrode used for selective electrochemical detection of mercury ions. Microporous Mesoporous Mater. 292, 109770 (2020)
A.J. Ahammad, J.-J. Lee, M. Rahman, Electrochemical sensors based on carbon nanotubes. Sensors 9(4), 289–2319 (2009)
S. Lü, Electrochemical determination of 8-azaguanine in human urine at a multi-carbon nanotubes modified electrode. Microchem. J. 77(1), 37–42 (2004)
C. Hu, S. Hu, Carbon nanotube-based electrochemical sensors: principles and applications in biomedical systems. J. Sens. (2009). https://doi.org/10.1155/2009/187615
G.A. Rivas et al., Carbon nanotubes for electrochemical biosensing. Talanta 74(3), 291–307 (2007)
Z. Amirzadeh, S. Javadpour, M.H. Shariat, R. Knibbe, Non-enzymatic glucose sensor based on copper oxide and multi-wall carbon nanotubes using PEDOT: PSS matrix. Synth. Met. 245, 160–166 (2018)
L. Hou et al., CO gas sensors based on p-type CuO nanotubes and CuO nanocubes: morphology and surface structure effects on the sensing performance. Talanta 188, 41–49 (2018)
Q. Zhang et al., CuO nanostructures: synthesis, characterization, growth mechanisms, fundamental properties, and applications. Prog. Mater. Sci. 60, 208–337 (2014)
J. Wu, W. Wang, M. Wang, H. Liu, H. Pan, Electrochemical behavior and direct quantitative determination of tanshinone IIA in micro-emulsion. Int. J. Electrochem. Sci. 11(6), 5165–5179 (2016). https://doi.org/10.20964/2016.06.55
E. Laviron, L. Roullier, C. Degrand, A multilayer model for the study of space distributed redox modified electrodes: Part II. Theory and application of linear potential sweep voltammetry for a simple reaction. J. Electroanal. Chem. Interfacial Electrochem. 112(1), 11–23 (1980)
N.M. Thanh, N.D. Luyen, T.T.T. Toan, N.H. Phong, N. Van Hop, Voltammetry determination of Pb(II), Cd(II), and Zn(II) at Bismuth Film electrode combined with 8-hydroxyquinoline as a complexing agent. J. Anal. Methods Chem. (2019). https://doi.org/10.1155/2019/4593135
E. Desimoni, B. Brunetti, Presenting analytical performances of electrochemical sensors. some suggestions. Electroanalysis 25(7), 1645–1651 (2013). https://doi.org/10.1002/elan.201300150
R. Madhu, K.V. Sankar, S.-M. Chen, R.K. Selvan, Eco-friendly synthesis of activated carbon from dead mango leaves for the ultrahigh sensitive detection of toxic heavy metal ions and energy storage applications. Rsc Adv. 4(3), 1225–1233 (2014)
X. Zhu et al., Alkaline intercalation of Ti3C2 MXene for simultaneous electrochemical detection of Cd (II), Pb (II), Cu (II) and Hg (II). Electrochim. Acta 248, 46–57 (2017)
H. Sopha et al., A new type of bismuth electrode for electrochemical stripping analysis based on the ammonium tetrafluorobismuthate bulk-modified carbon paste. Electroanalysis 22(13), 1489–1493 (2010)
R. Ouyang, Z. Zhu, C.E. Tatum, J.Q. Chambers, Z.-L. Xue, Simultaneous stripping detection of Zn (II), Cd (II) and Pb (II) using a bimetallic Hg–Bi/single-walled carbon nanotubes composite electrode. J. Electroanal. Chem. 656(1–2), 78–84 (2011)
H. Lin, G. Li, K. Wu, Electrochemical determination of Sudan I using montmorillonite calcium modified carbon paste electrode. Food Chem. 107(1), 531–536 (2008). https://doi.org/10.1016/j.foodchem.2007.08.022
C. Kokkinos, A. Economou, I. Raptis, C.E. Efstathiou, Lithographically fabricated disposable bismuth-film electrodes for the trace determination of Pb (II) and Cd (II) by anodic stripping voltammetry. Electrochim. Acta 53(16), 5294–5299 (2008)
Funding
No funding was received for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
AL-Refai, H.H., Ganash, A.A. & Hussein, M.A. Composite Nanoarchitectonics with Polythiophene, MWCNTs-G, CuO and Chitosan as a Voltammetric Sensor for Detection of Cd(II) Ions. J Inorg Organomet Polym 32, 713–727 (2022). https://doi.org/10.1007/s10904-021-02125-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-021-02125-8