Abstract
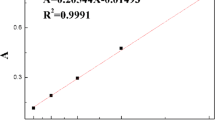
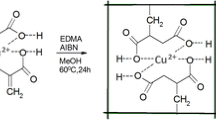
In this paper, Methacrylic acid (MAA) and 4-vinyl pyridine (4-VP) as functional monomers, Ethylene glycol two methyl acrylate (EGDMA) as crosslinking agent, isopropyl alcohol as the solvent, prepared the Cu(II)- and Pb(II)- imprinted polymers (IIPs) submicron spheres by precipitation polymerization. The presence/absence of the template ion in the preparation of the imprinted polymer was confirmed by EDX spectroscopy, and the structure of the particles was investigated using IR, SEM and BET analysis. From different components of crosslinker/monomer (C/M) ratio analysis, C/M at 1:3 was the optimal ratio for preparing IIPs. Atomic absorption spectroscopy (AAS) was characterized the imprinted polymers absorption behavior. The results show that the maximum adsorption capacity of Cu2+ and Pb2+ -imprinted polymer were 26.9 mg g−1 and 25.3 mg g−1, respectively. They also have good adsorption capacity and superior selectivity property for Cu2+ and Pb2+ in water, respectively. The selectivity factors (α) for Ni2+, Zn2+, Co2+ and Fe2+ were 16.5 (Cu2+) and 12.1 (Pb2+), 13.8 (Cu2+) and 16.2 (Pb2+), 10.8 (Cu2+) and 10.1 (Pb2+), 20.4 (Cu2+) and 20.7 (Pb2+), respectively. The regeneration experiment result demonstrates an excellent re-utilization property of these two type IIPs, after ten uses, the adsorption capacity can maintain above 60%.










Similar content being viewed by others
References
N.N. Dil, M. Sadeghi, J. Hazard. Mater. 351, 38 (2018)
L. Elci, M. Dogan, Fresen. J. Anal. Chem. 330, 610 (1988)
E. Carasek, Talanta 51, 173 (2000)
M.L. Tummino, R. Nistico, C. Riedo, D. Fabbri, G. Magnacca, Chem-Eur. J. 27, 660 (2020)
D. Wang, B. Zhang, L.F. Xu, L.N. Huang, Bull. Chem. Soc. Jpn. 93, 92 (2020)
O. Almeida, R.M. Menezes, L.S. Nunes, V.A. Lemos, F.G. Velasco, Environ. Technol. Innov. 21, 101336 (2020)
B. Unnikrishnan, C.W. Lien, H.W. Chu, H.W. Chu, H.W. Chu, J. Hazard. Mater. 401, 123397 (2020)
P.G. Krishna, J.M. Gladis, T.P. Rao, G.R. Naidu, J. Mol. Recog. 18, 109 (2005)
G. Wulff, A. Sarhan, Angew. Chem. Int. Ed. 11, 341 (1972)
H.H. Yang, S.Q. Zhang, W. Yang, X.L. Chen, Z.X. Zhang, J.G. Xu, X.R. Wang, J. Am. Chem. Soc. 126, 4054 (2004)
A. Ma, A. Ms, B. Bs, J. Electroanal. Chem. 879, 114788 (2020)
M. Saraji, H. Yousefi, J. Hazard. Mater. 167, 1152 (2009)
D.K. Singh, S. Mishra, J. Hazard. Mater. 164, 1547 (2009)
D.K. Singh, S. Mishra, Desalination 257, 177 (2010)
G. Bayramoglu, M.Y. Arica, J. Hazard. Mater. 187, 213 (2011)
G.M. Murray, K.A. Van Houten, G.L. Southard 2007 WO Patent 2007/055767A1.
A. Bhaskarapillai, N.V. Sevilimedu, B. Sellergren, Ind. Eng. Chem. Res. 48, 3730 (2009)
V.M. Biju, J.M. Gladis, T.P. Rao, Anal. Chim. Acta 478, 43 (2003)
M.R. Ganjali, A. Ghesmi, M. Hosseini, M.R. Pourjavid, M. Rezapour, M. Shamsipur, M.S. Niasari, Sens. Actuators B 105, 334 (2005)
M.R. Ganjali, L. Naji, T. Poursaberi, M. Shamsipur, S. Haghgoo, Anal. Chim. Acta 475, 59 (2003)
M.R. Ganjali, M.R. Pourjavid, M. Rezapour, S. Haghgoo, Sens. Actuators B 89, 21 (2003)
M.R. Ganjali, A. Daftari, M. Rezapour, T. Puorsaberi, S. Haghgoo, Talanta 59, 613 (2003)
M. Shamsipur, M. Yousefi, M.R. Ganjali, Anal. Chem. 72, 2391 (2000)
Y. Jiang, D. Kim, Chem. Eng. J. 166, 435 (2011)
Y. Jiang, D. Kim, Polym. Adv. Technol. 24, 747 (2013)
M. Kim, Y. Jiang, D. Kim, React. Funct. Polym. 73, 821 (2013)
Y. Jiang, D. Kim, Chem. Eng. J. 232, 503 (2013)
Y. Jiang, D. Kim, J. Nanosci. Nanotechnol. Revisions 14, 8578 (2014)
Y. Jiang, D. Kim, Ind. Eng. Chem. Res. 53, 13340 (2014)
N.T. Hoai, D. Kim, AIChE J. 55, 3248 (2009)
N.T. Hoai, D.K. Yoo, D. Kim, J. Hazard. Mater. 173, 462 (2010)
R. Huang, N. Shao, L. Hou, X. Zhu, Colloid Surface A 566, 218 (2019)
W. Shen, X. Jiang, Q. An, Z.Y. Xiao, S.R. Zhai, L. Cui, New J. Chem. 43, 5495 (2019)
H. Wang, H. Shang, X. Sun, L. Hou, M. Wen, Y. Qiao, Colloid Surface A 585, 124139 (2020)
A. Bukhari, N.H. Elsayed, M. Monier, Int. J. Biol. Macromol. 155, 795 (2020)
S.D. Masi, A. Pennetta, A. Guerreiro, F. Canfarotta, C. Malitesta, Sensor Actuat B-Chem. 307, 127648 (2019)
B. Ara, M. Muhammad, T.U.Z. Rani, K. Gul, Desalin. Water. Treat. 191, 173 (2020)
Acknowledgements
This research was supported by Zhejiang Provincial Natural Science Foundation (No. LQ19E030014). National Natural Science Foundation of China (No. 81901900). National Natural Science Foundation of China (No. 51902135).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiang, Y., Tang, B., Zhao, P. et al. Synthesis of Copper and Lead Ion Imprinted Polymer Submicron Spheres to Remove Cu2+ and Pb2+. J Inorg Organomet Polym 31, 4628–4636 (2021). https://doi.org/10.1007/s10904-021-02065-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-021-02065-3