Abstract
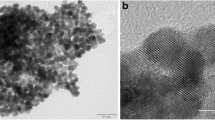
In this study, different ionic liquids including 1-aminoethyl-3-methyl-imidazolium bromide [C2NH2mim][Br] (1), 1-methyl-3-octylimidazolium chloride [C8mim][Cl] (2) and 1-methyl-3-octylimidazolium tetrafluoroborate [C8mim][BF4] (3) were applied to stabilize Pd nanoparticles (NPs) at toluene/water interface as thin films. Field emission-scanning electron microscopy (FE-SEM) images showed puckered chains of ionic liquid (1) around the Pd NPs as flower nanostructures. Transmission electron microscopy (TEM) image of Pd/1 showed clearly core–shell nanostructures. Furthermore, applications of Pd/1, Pd/2 and Pd/3 were investigated in the Suzuki–Miyaura C–C coupling reaction in the presence and absence of ultrasonic waves, hydrogenation catalysis of p-nitrophenol reduction and methanol oxidation reaction. Interestingly, Jf (forwarding current density due to methanol oxidation) was observed only for Pd/1. We believe that interactions of –NH2 and imidazolium groups of ionic liquid 1 with Pd particles are very important in producing of puckered shells around the Pd NPs. Injection of electron density from –NH2 group and Br− of ionic liquid 1 to Pd content tends to Pd be softer than other ionic liquids (2 or 3). This effect weakens the strength of Pd–O and facilitates the intermolecular reductive elimination between Pd–O and Pd–C≡O in rate-determining step of methanol oxidation to produce CO2 product. However, electron releasing group accelerates the increasement in negative charge density of metal accelerates intramolecular or intermolecular reduction elimination.
Graphic Abstract
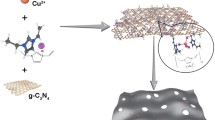
Stabilization of Pd nanoparticles from organometallic precursor in the presence of various ionic liquids is investigated. The obtained Pd/ionic liquid thin films were applied in catalytic reactions such as Suzuki–Miyaura C–C coupling reaction, p-nitrophenol reduction and methanol oxidation process for fuel cells. In spite of Pd nanoparticle thin films used for methanol oxidation reaction that exhibit no considerable methanol oxidation, these catalysts exhibit good electrocatalytic activity.






























Similar content being viewed by others
References
S.M. Navarro Gallon, E. Alpaslan, M. Wang, P. Larese-Casanova, M.E. Londono, L. Atehortua, J.J. Pavon, T.J. Webster, Mater. Sci. Eng. C 99, 685–695 (2019)
ASh Shamsabadi, H. Tavanai, M. Ranjbar, M. Bazarganipour, J. Inorg. Organomet. Polym. Mater. 30, 695–705 (2020)
M. Chauhan, C. Feizullayeva, K. Melepura, S. Matam, A. Patel, Q. Johnson, B.P.S. Chauhan, J. Inorg. Organomet. Polym. Mater. 24, 994–1000 (2014)
A. Miri, M. Khatami, M. Sarani, J. Inorg. Organomet. Polym. Mater. 30, 767–774 (2020)
T. Amaya, T. Isaji, M. Abe, T. Hirao, J. Inorg. Organomet. Polym. Mater. 25, 145–152 (2015)
J. Polte, Cryst. Eng. Comm. 17, 6809–6830 (2015)
X. Chen, M. Cheng, D. Chen, R. Wang, A.C.S. Appl, Mater. Interfaces 8, 3892–3900 (2016)
K. Piradashvili, E.M. Alexandrino, F.R. Wurm, K. Landfester, Chem. Rev. 116, 2141–2169 (2016)
F. Ozel, E. Aslan, A. Sarilmaz, I.H. Patir, A.C.S. Appl, Mater. Interfaces 8, 25881–25887 (2016)
S.G. Booth, R.A.W. Dryfe, J. Phys. Chem. C 119, 23295–23309 (2015)
S.J. Hoseini, M. Rashidi, M. Bahrami, J. Mater. Chem. 21, 16170–16176 (2011)
S.J. Hoseini, M. Dehghani, H. Nasrabadi, Catal. Sci. Technol. 4, 1078–1083 (2014)
S. Saberi Sarmoor, S.J. Hoseini, R. Hashemi Fath, M. Roushani, M. Bahrami, Appl. Organomet. Chem. 32, e3979–e3987 (2018)
S.J. Hoseini, N. Mousavi, M. Roushani, L. Mosadeghi, M. Bahrami, M. Rashidi, Dalton Trans. 42, 12364–12369 (2013)
S.J. Hoseini, M. Bahrami, M. Zanganeh, M. Roushani, M. Rashidi, RSC Adv. 6, 45753–45767 (2016)
S.J. Hoseini, M. Bahrami, M. Roushani, RSC Adv. 4, 46992–46999 (2014)
Z.H. Han, B. Yang, Y. Qi, J. Cumings, Ultrasonics 51, 485–488 (2011)
J. Kundu, D. Pradhan, A.C.S. Appl, Mater. Interfaces 6, 1823–1834 (2014)
B.P. Binks, Colloidal Particles at Liquid Interfaces (Cambridge University Press, New York, 2006)
A.S. Kabalnov, E.D. Shchukin, Adv. Colloid Interface Sci. 38, 69–97 (1992)
A. Glotov, A. Stavitskaya, Y. Chudakov, E. Ivanov, W. Huang, V. Vinokurov, A. Zolotukhina, A. Maximov, E. Karakhanov, Y. Lvov, Bull. Chem. Soc. Jpn. 92, 61–69 (2019)
R. Schomaecker, R. Strey, J. Phys. Chem. 98, 3908–3912 (1994)
C. Janiak, Catalysis in Ionic Liquids: From Catalyst Synthesis to Application (Science, Germany, 2014)
F. Han, S. Li, R. Yin, H. Liu, L. Xu, Colloids Surf. A 315, 210–216 (2008)
Y. Zhou, J. Qu, A.C.S. Appl, Mater. Interfaces 9, 3209–3222 (2017)
X. Zhong, Z. Liu, D. Cao, J. Phys. Chem. B 115, 10027–10040 (2011)
S. Zhang, Y. Zhang, Y. Wang, S. Liu, Y. Deng, Phys. Chem. Chem. Phys. 14, 5132–5138 (2012)
A. Biffis, M. Zecca, M. Basato, J. Mol. Catal. A 173, 249–274 (2001)
M. Perez-Lorenzo, J. Phys. Chem. Lett. 3, 167–174 (2012)
T.H. Rehm, A. Bogdan, C. Hofmann, P. Lob, Z.B. Shifrina, D.G. Morgan, L.M. Bronstein, ACS Appl. Mater. Interfaces 7, 27254–27261 (2015)
T. Swathi, G. Buvaneswari, Mater. Lett. 62, 3900–3902 (2008)
C. Huang, W. Ye, Q. Liu, X. Qiu, ACS Appl. Mater. Interfaces 6, 14469–14476 (2014)
D. Formenti, F. Ferretti, F.K. Scharnagl, M. Beller, Chem. Rev. 119, 2611–2680 (2019)
S. Zhong, Q. Xu, Bull. Chem. Soc. Jpn. 91, 1606–1617 (2018)
X. Zhang, J. Zhu, C.S. Tiwary, Z. Ma, H. Huang, J. Zhang, Z. Lu, W. Huang, Y. Wu, ACS Appl. Mater. Interfaces 8, 10858–10865 (2016)
S. Fu, C. Zhu, D. Du, Y. Lin, ACS Appl. Mater. Interfaces 8, 6110–6116 (2016)
J.N. Tiwari, W.G. Lee, S. Sultan, M. Yousuf, A.M. Harzandi, V. Vij, K.S. Kim, ACS Nano 11, 7729–7735 (2017)
H. You, F. Zhang, Z. Liu, J. Fang, ACS Catal. 4, 2829–2835 (2014)
N. Mansor, T.S. Miller, I. Dedigama, A.B. Jorge, J. Jia, V. Brazdova, C. Mattevi, C. Gibbs, D. Hodgson, P.R. Shearing, C.A. Howard, F. Cora, M. Shaffer, D.J.L. Brett, P.F. McMillan, Electrochim. Acta 222, 44–57 (2016)
Z. Yang, X. Zhou, H. Nie, Z. Yao, S. Huang, ACS Appl. Mater. Interfaces 3, 2601–2606 (2011)
D.H. Nagaraju, S. Devaraj, P. Balaya, Mater. Res. Bull. 60, 150–157 (2014)
R.K. Pandey, V. Lakshminarayanan, J. Phys. Chem. C 113, 21596–21603 (2009)
Z. Yin, H. Zheng, D. Ma, X. Bao, J. Phys. Chem. C 113, 1001–1005 (2009)
S.J. Hoseini, M. Bahrami, M. Dehghani, RSC Adv. 4, 13796–13804 (2014)
A.P. Ginsberg, Inorganic Syntheses (Inorganic Syntheses, Inc., New Jersey, 1990)
X. Zhou, T. Wu, K. Ding, B. Hu, M. Hou, B. Han, Chem. Commun. 14, 1897–1899 (2009)
M.H. Ghatee, A.R. Zolghadr, Fluid Phase Equilib. 263, 168–175 (2008)
X. Zhou, Y. Zhang, Z. Huang, D. Lu, A. Zhu, G. Shi, Nat. Sci. Rep. 6, 1–10 (2016)
H. Yang, C. Shan, F. Li, D. Han, Q. Zhang, L. Niu, Chem. Comm. 26, 3880–3882 (2009)
T. Rajkumar, G.R. Rao, Mater. Chem. Phys. 112, 853–857 (2008)
M. Bahrami, S.J. Hoseini, Appl. Organomet. Chem. 32, e3920–e3931 (2018)
A. Balanta, C. Godard, C. Claver, Chem. Soc. Rev. 40, 4973–4985 (2011)
J. Xiang, P. Li, H. Chong, L. Feng, F. Fu, Z. Wang, S. Zhang, M. Zhu, Nano Res. 7, 1337–1343 (2014)
Z.L. Du, Q.Q. Dang, X.M. Zhang, Ind. Eng. Chem. Res. 56, 4275–4280 (2017)
M. Nasrollahzadeh, B. Jaleh, A. Ehsania, New J. Chem. 39, 1148–1153 (2015)
S. Shabbir, S. Lee, M. Lim, H. Lee, H. Ko, Y. Lee, H. Rhee, J. Organomet. Chem. 846, 296–304 (2017)
N. Shang, C. Feng, H. Zhang, S. Gao, R. Tang, C. Wang, Z. Wang, Catal. Commun. 40, 111–115 (2013)
S. Liu, Q. Zhou, Z. Jin, H. Jiang, X. Jiang, Chin. J. Catal. 31, 557–561 (2010)
M. Nasrollahzadeh, S.M. Sajadi, A. Rostami-Vartooni, M. Khalaj, J. Mol. Catal. A 396, 31–39 (2015)
R. Zhang, J. Liu, F. Li, S. Wu, C. Xia, W. Sun, Chin. J. Chem. 29, 525–530 (2011)
Y.V. Ioni, S.E. Lyubimov, A.A. Korlyukov, M.Y. Antipin, V.A. Davankov, S.P. Gubin, Russ. Chem. Bull. 61, 1825–1827 (2012)
R. Kumar Rai, K. Gupta, S. Behrens, J. Li, Q. Xu, S. Kumar Singh, ChemCatChem 7, 1806–1812 (2015)
Ö. Metin, S. Fae Ho, C. Alp, H. Can, M.N. Mankin, M. Serdar Gültekin, M. Chi, S. Sun, Nano Res. 6, 10–18 (2013)
V. Calo, A. Nacci, A. Monopoli, F. Montingelli, J. Org. Chem. 70, 6040–6044 (2005)
N. Ghanbari, S.J. Hoseini, M. Bahrami, Ultrason. Sonochem. 39, 467–477 (2017)
D. Kaleeswaran, R. Antony, A. Sharma, A. Malani, R. Murugavel, ChemPlusChem 82, 1253–1265 (2017)
S.J. Hoseini, M. Bahrami, N. Sadri, N. Aramesh, Z. Samadi Fard, H. Rafatbakhsh Iran, B. Habib Agahi, M. Maddahfar, M. Dehghani, A. Zarei Baba Arabi, N. Heidari, S.F. Hashemi Fard, Z. Moradi, J. Colloid Interface Sci. 513, 602–616 (2018)
S. Lebaschi, M. Hekmati, H. Veisi, J. Colloid Interface Sci. 485, 223–231 (2017)
M. Nasrollahzadeh, S.M. Sajadi, A. Rostami-Vartooni, M. Bagherzadeh, J. Colloid Interface Sci. 448, 106–113 (2015)
Q. Liu, Y.R. Xu, A.J. Wang, J.J. Feng, RSC Adv. 5, 96028–96033 (2015)
S.K. Ghosh, M. Mandal, S. Kundu, S. Nath, T. Pal, Appl. Catal. 268, 61–66 (2004)
R. Antony, R. Marimuthu, R. Murugavel, ACS Omega 4, 9241–9250 (2019)
Y. Liu, Z. Liu, D. Huang, M. Cheng, G. Zeng, C. Lai, C. Zhang, C. Zhou, W. Wang, D. Jiang, H. Wang, B. Shao, Coord. Chem. Rev. 388, 63–78 (2019)
G. Law, P.R. Watson, Langmuir 17, 6138–6141 (2001)
D.H. Kwak, Y.W. Lee, K.H. Lee, A.R. Park, J.S. Moon, K.W. Park, Int. J. Electrochem. Sci. 8, 5102–5107 (2013)
H. Li, D. Kang, H. Wang, R. Wang, Int. J. Electrochem. Sci. 6, 1058–1065 (2011)
T. Maiyalagan, T.O. Alaje, K. Scott, J. Phys. Chem. C 116, 2630–2638 (2012)
J. Kramer, E. Redel, R. Thomann, C. Janiak, Organometallics 27, 1976–1978 (2008)
Acknowledgements
We thank Iran National Science Foundation (INSF) (Grant No. 96010235), Shiraz University Research Council and Yasouj University Council for their support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gheitasi Zarooni, M., Hoseini, S.J., Bahrami, M. et al. Pd/[C2NH2mim][Br] Thin Film Versus Pd/[C8mim][Cl] or Pd/[C8mim][BF4]: Catalytic Applications in Electrooxidation of Methanol, p-Nitrophenol Reduction and C–C Coupling Reaction. J Inorg Organomet Polym 30, 3448–3475 (2020). https://doi.org/10.1007/s10904-020-01514-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-020-01514-9