Abstract
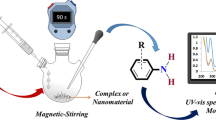
Schiff base Cu(II), Co(II), Ni(II), Cr(III) and Fe(III) complexes have been synthesized via the reaction of the 2-amino-3-hydroxy-4-bromopyridine with 2-chloro-5-nitrobenzaldehyde and different transition metal salts. The complexes have been characterized by several methods. In addition, the prepared complexes were tested as precursors for the synthesis of their corresponding metal oxides nanoparticles by controlled aerobic thermal decomposition at 500 °C. The structure, composition, morphology and particle size of the prepared nanostructured metal oxides were characterized by FTIR, X-ray powder diffraction and by scanning and transmission electron microscopy. In addition, the corresponding materials have been tested as catalysts in the heterogeneous selective “solvent free” oxidation of benzyl alcohol giving high selectivities to benzaldehyde. In addition, the nanostructured metal oxides gave, in general, higher conversions than those of the starting Schiff base metal complexes. Finally, the nanostructured metal oxides have been treated with an organometallic Pd(II) precursor to prepare Pd-doped metal oxides, which have been tested as catalysts in two different C–C coupling reactions, namely, the coupling reaction between iodobenzene and phenylboronic acid and the double coupling of iodobenzene with 2,6-dibromopyridin. In all cases interesting conversions and yields were observed for the nanostructured metal oxides.
Graphic Abstract












Similar content being viewed by others
References
X. Li, Z. Liu, Y. Xu, D. Wang, J. Inorg. Biochem. 171, 37 (2017)
J.L. Segura, M.J. Mancheño, F. Zamora, Chem. Soc. Rev. 45, 5635 (2016)
A.M. Abu-Dief, I.M.A. Mohamed, Beni-Suef Univ. J. Basic Appl. Sci. 4, 119 (2015)
A.A.A. Aziz, A.N.M. Salem, M.A. Sayed, M.M. Aboaly, J. Mol. Struct. 1010, 130–138 (2012)
B.S. Kusmariya, A.P. Mishra, J. Mol. Struct. 1130, 727 (2017)
P. Bhowmik, N. Aliaga-Alcalde, V. Gómez, M. Corbella, S. Chattopadhyay, Polyhedron 49, 269 (2013)
T. Mukherjee, J.C. Pessoa, A. Kumar, A.R. Sarkar, Dalton Trans. 42, 2594 (2013)
L.H. Abdel-Rahman, R.M. El-Khatib, L.A.E. Nassr, A.M. Abu-Dief, M. Ismael, A.A. Seleem, Spectrochim. Acta Part A 117, 366 (2014)
R.B. Sumathi, M.B. Halli, Bioinorg. Chem. Appl. 2014, 942162 (2014)
M.S. El-Shahawi, M.S. Al-Jahdali, A.S. Bashammakh, A.A. Al-Sibaai, H.M. Nassef, Spectrochim. Acta Part A 113, 459 (2013)
J. Zhang, F. Zhao, X. Zhu, W.-K. Wong, D. Ma, W.-Y. Wong, J. Mater. Chem. 22, 16448 (2012)
R. Drozdzak, B. Allaert, N. Ledoux, I. Dragutan, V. Dragutan, F. Verpoort, Adv. Synth. Catal. 347, 1721 (2005)
K.C. Emregül, E. Düzgün, O. Atakol, Corros. Sci. 48, 3243 (2006)
L.H.A. Rahman, A.M. Abu-Dief, R.M. El-Khatib, S.M. Abdel-Fatah, A.M. Adam, E.M.M. Ibrahim, Appl. Organomet. Chem. 32, e4174 (2018)
L.H. Abdel-Rahman, A.M. Abu-Dief, R.M. El-Khatib, S.M. Abdel-Fatah, J. Photochem. Photobiol. 162, 298 (2016)
A. Bhaumik, R. Kumar, J. Chem. Soc., Chem. Commun. 3, 349–350 (1995)
A. Didi, L.M. Gómez-Calcerrada, A. Benhamou, S. Gómez-Ruiz, Ceram. Int. 44, 17266 (2018)
P. Mondal, P. Banja, R. Khatun, A. Bhaumik, D. Das, S.M. Islam, J. Colloid Interface Sci. 508, 378–386 (2017)
M.R. Bermejo, A.M. González, M. Fondo, M. Maneiro, M. Rey, M. Vázquez, O.L. Hoyos, J.C. Garcı́a-Monteagudo, Inorg. Chim. Acta 307, 106–114 (2000)
Z. Quan, E. Ni, Y. Ogasawara, N. Sonoyama, Solid State Ionics 262, 128 (2014)
A. Balbín, F. Gaballo, J. Ceballos-Torres, S. Prashar, M. Fajardo, G.N. Kaluđerović, S. Gómez-Ruiz, RSC Adv. 4, 54775 (2014)
R.S. Erami, D. Díaz-García, S. Prashar, A. Rodríguez-Diéguez, M. Fajardo, M. Amirnasr, S. Gómez-Ruiz, Catalysts 7, 76 (2017)
S. Lázaro-Navas, S. Prashar, M. Fajardo, S. Gómez-Ruiz, J. Nanopart. Res. 17, 94 (2015)
B. Rico-Oller, A. Boudjemaa, H. Bahruji, M. Kebir, S. Prashar, K. Bachari, M. Fajardo, S. Gómez-Ruiz, Sci. Total Environ. 563–564, 921 (2016)
A.M.S. Hossain, A. Balbín, R.S. Erami, S. Prashar, M. Fajardo, S. Gómez-Ruiz, Inorg. Chim. Acta 455, 645 (2017)
James Keeler, Understanding NMR spectroscopy, 2nd edn. (Wiley, Chichester, 2010)
H.-Q. Huang, X.-Y. Cheng, T. Zhang, R.-B. Huang, Inorg. Chem. Commun. 68, 21 (2016)
M. Salehi, F. Rahimifar, M. Kubicki, A. Asadi, Inorg. Chim. Acta 443, 28 (2016)
S.K. Sawant, G.A. Gaikwad, V.A. Sawant, B.A. Yamgar, S.S. Chavan, Inorg. Chem. Commun. 12, 632 (2009)
M.B. Halli, R.B. Sumathi, J. Mol. Struct. 1022, 130 (2012)
M. Montazerozohori, S.M. Jahromi, A. Naghiha, J. Ind. Eng. Chem. 22, 248 (2015)
S.M. Pradeepa, H.B. Naik, B.V. Kumar, K.I. Priyadarsini, A. Barik, T.R. Naik, M.C. Prabhakara, Spectrochim. Acta Part A 115, 12–21 (2013)
A. Hauser, in Spin Crossover, in Transition metal compounds I, ed. by P. Gütlich, H.A. Goodwin (Springer, Berlin, 2004), pp. 49–58
T. Munisamy, S.L. Gipson, J. Organomet. Chem. 692, 1087 (2007)
W. Tang, Y. Deng, W. Li, S. Li, X. Wu, Y. Chen, Catal. Commun. 72, 165 (2015)
S. Srivastava, IOSR-JAP 5, 61 (2013)
M.A. Shah, M.S. Al-Ghamdi, MSA 02, 977 (2011)
D. DurgaVijaykarthik, M. Kirithika, N. Prithivikumaran, N. Jeyakumaran, Int. J. Nano Dimens. 5, 557 (2014)
P.V.R.K. Ramacharyulu, S.J. Abbas, S.R. Sahoo, S.-C. Ke, Catal. Sci. Technol. 8, 2825 (2018)
D. Díaz-García, P.R. Ardiles, S. Prashar, A. Rodríguez-Diéguez, P.L. Páez, S. Gómez-Ruiz, Pharmaceutics 11, 30 (2019)
M. Hosseini-Sarvari, Z. Razmi, Helv. Chim. Acta 98, 805 (2015)
Acknowledgements
We gratefully acknowledge financial support of Ministerio de Ciencia, Innovación y Universidades of Spain (Grant No. RTI2018-094322-B-I00).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abdel-Fatah, S.M., Díaz-Sánchez, M., Díaz-García, D. et al. Nanostructured Metal Oxides Prepared from Schiff Base Metal Complexes: Study of the Catalytic Activity in Selective Oxidation and C–C Coupling Reactions. J Inorg Organomet Polym 30, 1293–1305 (2020). https://doi.org/10.1007/s10904-019-01269-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-019-01269-y