Abstract
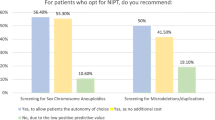
Since its introduction four years ago, noninvasive prenatal screening for fetal aneuploidy (NIPS) has been widely adopted as a screening tool for women at a high risk for fetal aneuploidy. As use expands into the general population, questions arise concerning the integration of NIPS into preexisting screening paradigms. This study aims to examine the use of NIPS in current practice among prenatal counselors, predominantly in the United States, in order to inform strategies for the optimal use of both new and existing screening techniques. We electronically surveyed 208 members of the National Society of Genetic Counselors to ascertain how NIPS is currently being used. Genetic counselors were also queried as to the advantages and disadvantages of offering NIPS to all patients regardless of a priori risk. Results indicate substantial variation in practice regarding which patients are offered NIPS and how counselors have incorporated this technology into existing screening routines. The majority of participants report offering NIPS in conjunction with another method of screening for fetal aneuploidy, indicating that NIPS is being used as an addition rather than as a replacement. These screening methods primarily include nuchal translucency (NT) (45.1 %, n = 78) and first trimester serum screening, with or without an NT (19.7 %, n = 34). Furthermore, the majority report that they would be concerned about losing the clinical value of an NT in a complete transition to NIPS (85.4 %, n = 164). Counselors are evenly split on the merits of expanding the use of NIPS to the general population (con: 55.3 %, n = 105; pro: 44.7 %, n = 85). The lack of consensus suggests that updated practice guidelines might benefit counselors. In addition, respondents emphasized the need to better educate patients and providers about the risks, benefits, and limitations of the test.



Similar content being viewed by others
References
Abu-rustum, R. S., Daou, L., & Abu-rustum, S. E. (2010). Role of first-trimester sonography. Journal of Ultrasound in Medicine, 29, 1445–1452.
Allyse, M., Minear, M. A., Berson, E., Sridhar, S., Rote, M., Hung, A., & Chandrasekharan, S. (2015). Non-invasive prenatal testing: a review of international implementation and challenges. International Journal of Women’s Health, 7, 113–126. doi:10.2147/IJWH.S67124.
American College of Obstetricians and Gynecologists (2012). Noninvasive prenatal testing for fetal aneuploidy. Committee opinion No. 545. Obstetrics & Gynecology, 120, 1532–1534.
American College of Obstetricians and Gynecologists (2015). Cell-free DNA screening for fetal aneuploidy. Committee Opinion No. 640. Obstetrics & Gynecology. doi:10.1097/AOG.0000000000001007.
Ashoor, G., Syngelaki, A., Poon, L. C. Y., Rezende, J. C., & Nicolaides, K. H. (2013). Fetal fraction in maternal plasma cell-free DNA at 11-13 weeks’ gestation: relation to maternal and fetal characteristics. Ultrasound in Obstetrics & Gynecology, 41, 26–32. doi:10.1002/uog.12331.
Atzei, A., Gajewska, K., Huggon, I. C., Allan, L., & Nicolaides, K. H. (2005). Relationship between nuchal translucency thickness and prevalence of major cardiac defects in fetuses with normal karyotype. Ultrasound in Obstetrics & Gynecology, 26(2), 154–157. doi:10.1002/uog.1936.
Begleiter, M. L., & Finley, B. E. (2014). Positive predictive value of cell free DNA analysis. American Journal of Obstetrics and Gynecology, 211(July), 81. doi:10.1016/j.ajog.2014.01.014.
Benn, P., Cuckle, H., & Pergament, E. (2013). Non-invasive prenatal testing for aneuploidy: current status and future prospects. Ultrasound in Obstetrics & Gynecology, 42(1), 15–33. doi:10.1002/uog.12513.
Benn, P., Borrell, A., Chiu, R., Cuckle, H., Dugoff, L., Faas, B., & Yaron, Y. (2015a). Position statement from the chromosome abnormality screening committee on behalf of the board of the international society for prenatal diagnosis. Prenatal Diagnosis. doi:10.1002/pd.4608.
Benn, P., Curnow, K. J., Chapman, S., Michalopoulos, S. N., Hornberger, J., & Rabinowitz, M. (2015b). An economic analysis of cell-free DNA non- invasive prenatal testing in the US general pregnancy population. PloS One, 10(7), 1–12. doi:10.1371/journal.pone.0132313.
Bianchi, D. W., Sehnert, A. J., & Rava, R. P. (2012). Genome-wide fetal aneuploidy detection by maternal plasma dna sequencing. Obstetrics & Gynecology, 119(6), 1270–1271. doi:10.1097/AOG.0b013e318258c419.
Bianchi, D. W., Parker, R. L., Wentworth, J., Madankumar, R., Saffer, C., Das, A. F., & Sehnert, A. J. (2014). DNA sequencing versus standard prenatal aneuploidy screening. New England Journal of Medicine, 370(9), 799–808. doi:10.1056/NEJMoa1311037.
Chetty, S., Garabedian, M. J., & Norton, M. E. (2013). Uptake of noninvasive prenatal testing (NIPT) in women following positive aneuploidy screening. Prenatal Diagnosis, 33(6), 542–546. doi:10.1002/pd.4125.
Dan, S., Wang, W., Ren, J., Li, Y., Hu, H., Xu, Z., & Zhang, X. (2012). Clinical application of massively parallel sequencing-based prenatal noninvasive fetal trisomy test for trisomies 21 and 18 in 11105 pregnancies with mixed risk factors. Prenatal Diagnosis, 32(13), 1225–1232. doi:10.1002/pd.4002.
Dar, P., Curnow, K. J., Gross, S. J., Hall, M. P., Stosic, M., Demko, Z., & Benn, P. (2014). Clinical experience and follow-up with large scale single-nucleotide polymorphism-based non-invasive prenatal aneuploidy testing. American Journal of Obstetrics and Gynecology, 211(5), 527.e1–527.e17. doi:10.1016/j.ajog.2014.08.006.
Devers, P. L., Cronister, A., Ormond, K. E., Facio, F., Brasington, C. K., & Flodman, P. (2013). Noninvasive prenatal testing/noninvasive prenatal diagnosis: the position of the national society of genetic counselors. Journal of Genetic Counseling, 22(3), 291–295. doi:10.1007/s10897-012-9564-0.
Dugoff, L. (2010). First- and second-trimester maternal serum markers for aneuploidy and adverse obstetric outcomes. Obstetrics & Gynecology, 115(5), 1052–1061. doi:10.1097/AOG.0b013e3181da93da.
Ehrich, M., Deciu, C., Zwiefelhofer, T., Tynan, J. A., Cagasan, L., Tim, R., & van den Boom, D. (2011). Noninvasive detection of fetal trisomy 21 by sequencing of DNA in maternal blood: a study in a clinical setting. American Journal of Obstetrics and Gynecology, 204(3), 205.e1–205.e11. doi:10.1016/j.ajog.2010.12.060.
Estreich, G. (2014). Consumer-directed advertising for noninvasive prenatal screening. Presented at the NSGC Annual Education Conference, New Orleans.
Fairbrother, G., Burigo, J., Sharon, T., Song, K., Fairbrother, G., Burigo, J., & Song, K. (2015). Prenatal screening for fetal aneuploidies with cell-free DNA in the general pregnancy population: a cost-effectiveness analysis. Journal of Maternal-Fetal and Neonatal Medicine, 7058(May), 1–5. doi:10.3109/14767058.2015.1038703.
First trimester screen | Fβ. (2015). Retrieved from http://ntdlabs.com/maternal-marker-testing/first_trimester_screen.php.
Freelon, D. (2013). ReCal OIR: Ordinal, interval, and ratio intercoder reliability as a web service. International Journal of Internet Science, 8(1), 10–16.
Gagnon, A., Wilson, R.D., Audibert, F., Allen, V.M., Blight, C., Brock, J.A., Désilets, V.A., Johnson, J.A., Langlois, S., Summers, A., & Wyatt, P. (2008). Obstetrical complications associated with abnormal maternal serum markers analytes. Journal of Obstetrics and Gynaecology Canada, 30(10), 918–949.
Gil, M. M., Quezada, M. S., Bregant, B., Ferraro, M., & Nicolaides, K. H. (2013). Implementation of maternal blood cell-free DNA testing in early screening for aneuploidies. Ultrasound in Obstetrics & Gynecology, 42(April), 34–40. doi:10.1002/uog.12504.
Grati, F. R., Malvestiti, F., Ferreira, J. C. P. B., Bajaj, K., Gaetani, E., Agrati, C., & Simoni, G. (2014). Fetoplacental mosaicism: potential implications for false-positive and false-negative noninvasive prenatal screening results. Genetics in Medicine, 16(8), 620–624. doi:10.1038/gim.2014.3.
Gregg, A. R., Gross, S. J., Best, R. G., Monaghan, K. G., Bajaj, K., Skotko, B. G., & Watson, M. S. (2013). ACMG statement on noninvasive prenatal screening for fetal aneuploidy. Genetics in Medicine, 15(5), 395–398. doi:10.1038/gim.2013.29.
Horsting, J. M. H., Dlouhy, S. R., Hanson, K., Quaid, K., Bai, S., & Hines, K. A. (2014). Genetic counselors’ experience with cell-free fetal DNA testing as a prenatal screening option for aneuploidy. Journal of Genetic Counseling, 23(3), 377–400. doi:10.1007/s10897-013-9673-4.
Kaimal, A. J., Norton, M. E., & Kuppermann, M. (2015). Prenatal testing in the genomic age clinical outcomes, quality of life, and costs. Obstetrics & Gynecology, 126(4), 737–746. doi:10.1097/AOG.0000000000001029.
Karow, J. (2014). Clinicians discuss NIPT vs invasive diagnostics, ethical issues at prenatal molecular dx conference. Retrieved from https://www.genomeweb.com/molecular-diagnostics/clinicians-discuss-nipt-vs-invasive-diagnostics-ethical-issues-prenatal.
Langlois, S., Brock, J. A, Wilson, R. D., Audibert, F., Carroll, J., Cartier, L., Senikas, V. (2013). Current status in non-invasive prenatal detection of down syndrome, trisomy 18, and trisomy 13 using cell-free DNA in maternal plasma. Journal of Obstetrics and Gynaecology Canada, 35(2), 177–181.
Larion, S., Warsof, S.L., Romary, L., Mlynarczyk, M., Peleg, D., Abuhamad, A.Z. (2014, March-April). Presented at the annual convention of the American Institute of Ultrasound in Medicine, Las Vegas.
Lee, W., Yagel, S., Cohen, S. M., Benacerraf, B. R., Cuckle, H., Kagan, K. O., & Wapner, R. (2015). Noninvasive Prenatal Testing and Fetal Sonographic Screening: Roundtable Discussion. Journal of Ultrasound in Medicine, 34(3), 363–369. doi:10.7863/ultra.34.3.363.
Lo, Y. M., Corbetta, N., Chamberlain, P. F., Rai, V., Sargent I. L., Redman C. W., et al. (1997). Presence of fetal DNA in maternal plasma and serum. Lancet, 350, 485–487.
Malone, F. D., Canick, J. A., Ball, R. H., Nyberg, D. A., Comstock, C. H., Bukowski, R., et al. (2005). First-trimester or second-trimester screening, or both, for Down’s syndrome. New England Journal of Medicine, 353(19), 2001–2011. doi:10.1056/NEJMoa1404304.
Maternal serum screening: product offerings. (2012). Retrieved from https://www.labcorp.com/wps/wcm/connect/intgeneticslib/IntegratedGenetics/Resources/PDFs/Brochures/maternal-serum-screening-physician-brochure.
Mennuti, M. T., Cherry, A. M., Morrissette, J. J. D., & Dugoff, L. (2013). Is it time to sound an alarm about false-positive cell-free DNA testing for fetal aneuploidy? American Journal of Obstetrics and Gynecology, 209(5), 415–419. doi:10.1016/j.ajog.2013.03.027.
Mennuti, M. T., Dugoff, L., Morrissette, J. J. D., & Cherry, A. M. (2014). Reply. American Journal of Obstetrics and Gynecology, 211(July), 81. doi:10.1016/j.ajog.2014.01.015.
National Society of Genetic Counselors (2015). NIPT/cfDNA calculator. Retrieved from https://secure.itswebs.com/nsgc/niptcalculator/index.html.
National Society of Genetic Counselors Prenatal Special Interest Group. (2015a). Abnormal prenatal cell-free DNA screening results. Retrieved from http://nsgc.org/page/abnormal-non-invasive-prenatal-testing-results.
National Society of Genetic Counselors Prenatal Special Interest Group. (2015b). Prenatal cell-free DNA screening. Retrieved from http://nsgc.org/page/non-invasive-prenatal-testing-healthcare-providers.
Nicolaides, K. H. (2004). Nuchal translucency and other first-trimester sonographic markers of chromosomal abnormalities. American Journal of Obstetrics and Gynecology, 191(1), 45–67. doi:10.1016/j.ajog.2004.03.090.
Nicolaides, K. H. (2011). Turning the pyramid of prenatal care. Fetal Diagnosis and Therapy, 29(3), 183–196. doi:10.1159/000324320.
Nicolaides, K. H., Syngelaki, A., Ashoor, G., Birdir, C., & Touzet, G. (2012). Noninvasive prenatal testing for fetal trisomies in a routinely screened first-trimester population. American Journal of Obstetrics and Gynecology, 207(5), 374.e1–374.e6. doi:10.1016/j.ajog.2012.08.033.
Nicolaides, K. H., Wright, D., Poon, L. C., Syngelaki, A., & Gil, M. M. (2013a). First-Trimester Contingent Screening for Trisomy 21 by Biomarkers and Maternal Blood Cell-Free DNA Testing. Ultrasound in Obstetrics & Gynecology, 42(1), 41–50. doi:10.1002/uog.12511.
Nicolaides, K. H., Syngelaki, A., Gil, M., Atanasova, V., & Markova, D. (2013b). Validation of targeted sequencing of single-nucleotide polymorphisms for non-invasive prenatal detection of aneuploidy of chromosomes 13, 18, 21, X, and Y. Prenatal Diagnosis, 33, 575–579. doi:10.1002/pd.4103.
Norton, M. E., Brar, H., Weiss, J., Karimi, A., Laurent, L. C., Caughey, A. B., & Song, K. (2012). Non-Invasive Chromosomal Evaluation (NICE) Study: Results of a multicenter prospective cohort study for detection of fetal trisomy 21 and trisomy 18. American Journal of Obstetrics and Gynecology, 207(2), 137.e1–137.e8. doi:10.1016/j.ajog.2012.05.021.
Norton, M. E., Jelliffe-Pawlowski, L. L., & Currier, R. J. (2014). Chromosome abnormalities detected by current prenatal screening and noninvasive prenatal testing. Obstetrics & Gynecology, 124, 979–986. doi:10.1097/AOG.0000000000000452.
Norton, M. E., Jacobsson, B., Swamy, G. K., Laurent, L. C., Ranzini, A. C., Brar, H., et al. (2015). Cell-free DNA analysis for noninvasive examination of trisomy. New England Journal of Medicine, 372(17), 1589–1597. doi:10.1056/NEJMoa1407349.
Palomaki, G. E., Kloza, E. M., Lambert-Messerlian, G. M., Haddow, J. E., Neveux, L. M., Ehrich, M., et al. (2011). DNA sequencing of maternal plasma to detect down syndrome: an international clinical validation study. Genetics in Medicine, 13(11), 913–920. doi:10.1097/GIM.0b013e3182368a0e.
Patton, M. Q. (2001). Qualitative evaluation and research methods. Thousand Oaks: Sage.
Pergament, E., Cuckle, H., Zimmermann, B., Banjevic, M., Sigurjonsson, S., Ryan, A., …Rabinowitz, M. (2014). Single-nucleotide polymorphism-based noninvasive prenatal screening in a high-risk and low-risk cohort. Obstetrics & Gynecology, 124(2 Pt 1), 210–218. doi:10.1097/AOG.0000000000000363.
Prenatal screening and diagnosis of neural tube defects, Down syndrome, and trisomy 18. (2015). Retrieved from https://www.labcorp.com/wps/wcm/connect/intgeneticslib/IntegratedGenetics/Resources/PDFs/Brochures/maternal-serum-screening-physician-brochure.
Quad screen | Fβ. (2015). Retrieved from http://ntdlabs.com/maternal-marker-testing/quad_screen.php.
Resta, R. (2014). NIPS SPIN [Blog post]. Retrieved from http://thednaexchange.com/2014/04/21/nips-spin/.
Royal Australian and New Zealand College of Obstetricians and Gynaecologists (2015). DNA-based noninvasive prenatal testing for fetal aneuploidy. Retrieved from https://www.ranzcog.edu.au/womens-health/college-communiques/1357-dna-based-noninvasive-prenatal-testing-for-fetal-aneuploidy.html.
Salomon, L. J., Alfirevic, Z., Bilardo, C. M., Chalouhi, G. E., Ghi, T., Kagan, K. O., & Yeo, G. (2013). ISUOG practice guidelines: performance of first-trimester fetal ultrasound scan. Ultrasound in Obstetrics & Gynecology, 41(1), 102–113. doi:10.1002/uog.12342.
Shulman, L. (2014). The science of pregnancy management: moving beyond NIPT and through the continuum of care. Presented at the ACMG Annual Clinical Genetics Meeting, Nashville.
Society for Maternal-Fetal Medicine (2015). Prenatal aneuploidy screening using cell-free DNA. SMFM Consult Series #36. American Journal of Obstetrics and Gynecology, 212(6), 711–716. doi:10.1016/j.ajog.2015.03.043.
Stoll, K. (2013a). NIPS and the threat to informed decision making [Blog post]. Retrieved from http://thednaexchange.com/2013/11/04/nips-and-the-threat-to-informed-decision-making/.
Stoll, K. (2013b). NIPS is not diagnostic – convincing our patients and convincing ourselves [Blog post]. Retrieved from http://thednaexchange.com/2013/07/11/guest-post-nips-is-not-diagnostic-convincing-our-patients-and-convincing-ourselves/.
Stoll, K. (2014a). Non-invasive prenatal screening: data, marketing, and women’s choices. presented at the NSGC Annual Education conference, New Orleans.
Stoll, K. (2014b). NIPS: microdeletions, macro questions [Blog post]. Retrieved from http://thednaexchange.com/2014/11/02/guest-post-nips-microdeletions-macro-questions/.
Stoll, K., & Lindh, H. (2015). The DNA exchange guest post : PPV puffery? Sizing up NIPT statistics [Blog post]. Retrieved from http://thednaexchange.com/2015/05/04/guest-post-ppv-puffery-sizing-up-nipt-statistics/.
Suskin Kaplan, B., Neto, N., Dar, P., Dolan, S. M., & Klugman, S. (2014). The value of the “double positive” first trimester screen. Nashville: Poster presented at the ACMG Annual Clinical Genetics Meeting.
Syngelaki, A., Chelemen, T., Dagklis, T., Allan, L., & Nicolaides, K. H. (2011). Challenges in the diagnosis of fetal non-chromosomal abnormalities at 11-13 Weeks. Prenatal Diagnosis, 31, 90–102. doi:10.1002/pd.2642.
Tamminga, S., van Schendel, R. V., Rommers, W., Bilardo, C. M., Pajkrt, E., Dondorp, W. J., van Maarle, M., Cornel, M. C., & Henneman, L. (2015). Changing to NIPT as a first-tier screening test and future perspectives: opinions of health professionals. Prenatal Diagnosis. doi:10.1002/pd.4697.
Walker, B. S., Nelson, R. E., Jackson, B. R., Grenache, D. G., Ashwood, R., & Schmidt, R. L. (2015). A Cost-effectiveness analysis of first trimester non-invasive prenatal screening for fetal trisomies in the united states. PloS One, 1–20. doi:10.1371/journal.pone.0131402.
Wilson, K. L., Czerwinski, J. L., Hoskovec, J. M., Noblin, S. J., Sullivan, C. M., Harbison, A., et al. (2013). NSGC practice guideline: prenatal screening and diagnostic testing options for chromosome aneuploidy. Journal of Genetic Counseling, 22(1), 4–15. doi:10.1007/s10897-012-9545-3.
Zhang, H., Gao, Y., Jiang, F., Fu, M., Yuan, Y., Guo, Y., & Wang, W. (2015). Non-invasive prenatal testing for trisomies 21, 18 and 13: clinical experience from 146,958 pregnancies. Ultrasound in Obstetrics & Gynecology, 45(5), 530–538. doi:10.1002/uog.14792.
Acknowledgments
This study was completed as part of the first author’s Master of Science degree. The authors wish to thank all those who assisted in the development of the survey, data analysis, and those who participated in the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict Of Interest
Authors Emily Suskin, Laura Hercher, Kathleen Erskine Aaron, and Komal Bajaj declare that they have no conflict of interest.
Human Studies and Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
Animal Studies
No animal studies were carried out by the authors for this article.
Rights and permissions
About this article
Cite this article
Suskin, E., Hercher, L., Aaron, K.E. et al. The Integration of Noninvasive Prenatal Screening into the Existing Prenatal Paradigm: a Survey of Current Genetic Counseling Practice. J Genet Counsel 25, 1032–1043 (2016). https://doi.org/10.1007/s10897-016-9934-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10897-016-9934-0