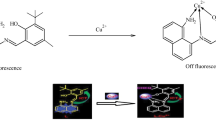
Abstract
A highly selective chemosensor BHC ((E)-N-benzhydryl-2-((2-hydroxynaphthalen-1-yl)methylene)hydrazine-1-carbothioamide) for detecting indium(III) was synthesized. Sensor BHC can detect In(III) by a fluorescence turn-on method. The detection limit was analyzed to be 0.89 μM. Importantly, this value is the lowest among those previously known for fluorescent turn-on In(III) chemosensors. Based on the analytical methods like ESI-mass, Job plot, and theoretical calculations, the detection mechanism for In(III) was illustrated to be chelation-enhanced fluorescence (CHEF) effect. Additionally, sensor BHC was successfully applied to test strips.









Similar content being viewed by others
References
Asami T, Yoshino A, Kubota M, Gotoh S (1990) Background level of indium and gallium in soil with special reference to the pollution of the soils from zinc and lead smelters. J Plant Nutr Soil Sci 153:257–259
Kho YM, Shin EJ (2017) Spiropyran-isoquinoline dyad as a dual chemosensor for Co(II) and In(III) detection. Molecules 22
Chen HW (2006) Gallium, indium, and arsenic pollution of groundwater from a semiconductor manufacturing area of Taiwan. Bull Environ Contam Toxicol 77:289–296
Han DY, Kim JM, Kim J, Jung HS, Lee YH, Zhang JF, Kim JS (2010) ESIPT-based anthraquinonylcalix[4]crown chemosensor for In3+. Tetrahedron Lett 51:1947–1951
Wu Y-C, Li H-J, Yang H-Z (2010) A sensitive and highly selective fluorescent sensor for In3+. Org Biomol Chem 8:3394–3397
Kim SK, Kim HS, Kim JH, Lee SH, Lee SW, Ko J, Bartsch RA, Kim JS (2005) indium(III)-induced fluorescent excimer formation and extinction in calix[4]arene-fluoroionophores. Inorg Chem 44:7866–7875
Kim H, Kim KB, Song EJ, Hwang IH, Noh JY, Kim PG, Jeong KD, Kim C (2013) Turn-on selective fluorescent probe for trivalent cations. Inorg Chem Commun 36:72–76
Acar O, Türker AR, Kılıç Z (1998) Determination of bismuth, indium and lead in geological samples by electrothermal AAS. Fresenius J Anal Chem 360:645–649
Gęca I, Korolczuk M (2017) Sensitive anodic stripping Voltammetric determination of indium(III) traces following double deposition and stripping steps. J Electrochem Soc 164:H183–H187
Adya VC, Kumar M, Sengupta A, Natarajan V (2015) Inductively coupled plasma atomic emission spectrometric determination of indium (In) and gallium (Ga) in thorium matrix after chemical separation using Cyanex 923 extractant. At Spectrosc 36:261–265
Tehrani MH, Companys E, Dago A, Puy J, Galceran J (2018) Free indium concentration determined with AGNES. Sci Total Environ 612:269–275
Lee JJ, Park GJ, Kim YS, Lee SY, Lee JH, Noh I, Kim C (2015) A water-soluble carboxylic-functionalized chemosensor for detecting Al3+ in aqueous media and living cells: experimental and theoretical studies. Biosens Bioelectron 69:226–229
He G, Meng Q, Zhao X, He C, Zhou P, Duan C (2016) A new copper(II) selective fluorescence probe based on naphthalimide: synthesis, mechanism and application in living cells. Inorg Chem Commun 65:28–31
Park GJ, Lee JJ, You GR, Nguyen L, Noh I, Kim C (2016) A dual chemosensor for Zn2+ and Co2+ in aqueous media and living cells: experimental and theoretical studies. Sensors Actuators B Chem 223:509–519
Wu D, Sedgwick AC, Gunnlaugsson T, Akkaya EU, Yoon J, James TD (2017) Fluorescent chemosensors: the past, present and future. Chem Soc Rev 46:7105–7123
Ghosh P, Banerjee P (2017) Small molecular probe as selective tritopic sensor of Al3+, F− and TNP: fabrication of portable prototype for onsite detection of explosive TNP. Anal Chim Acta 965:111–122
Huang L, Zhang J, Yu X, Ma Y, Huang T, Shen X, Qiu H, He X, Yin S (2015) A Cu2+-selective fluorescent chemosensor based on BODIPY with two pyridine ligands and logic gate. Spectrochim Acta A 145:25–32
Lim C, An M, Seo H, Huh JH, Pandith A, Helal A, Kim HS (2017) Fluorescent probe for sequential recognition of Ga3+ and pyrophosphate anions. Sensors Actuators B Chem 241:789–799
Kim DH, Im YS, Kim H, Kim C (2014) Solvent-dependent selective fluorescence sensing of Al3+ and Zn2+ using a single Schiff base. Inorg Chem Commun 45:15–19
Maity D, Govindaraju T (2011) Naphthaldehyde-urea/Thiourea conjugates as turn-on fluorescent probes for Al3+ based on restricted C=N isomerization. Eur J Inorg Chem 2011:5479–5485
Goswami S, Manna A, Paul S, Maity AK, Saha P, Quah CK, Fun H-K (2014) FRET based ‘red-switch’ for Al3+ over ESIPT based ‘green-switch’ for Zn2+ : dual channel detection with live-cell imaging on a dyad platform. RSC Adv 4:34572–34576
Jang HJ, Kang JH, Yun D, Kim C (2018) A multifunctional selective “turn-on” fluorescent chemosensor for detection of group IIIA ions Al3+, Ga3+ and In3+. Photochem Photobiol Sci 17:1247–1255. https://doi.org/10.1039/C8PP00171E
Hu J-H, Li J-B, Sun Y, Pei P-X, Qi J (2017) A turn-on fluorescent chemosensor based on acylhydrazone for sensing of Mg2+ with a low detection limit. RSC Adv 7:29697–29701
Magde D, Wong R, Seybold PG (2002) Fluorescence quantum yields and their relation to lifetimes of rhodamine 6G and fluorescein in nine solvents: improved absolute standards for quantum yields. Photochem Photobiol 75:327–334
Long L, Huang M, Wang N, Wu Y, Wang K, Gong A, Zhang Z, Sessler JL (2018) A mitochondria-specific fluorescent probe for visualizing endogenous hydrogen cyanide fluctuations in neurons. J Am Chem Soc 140:1870–1875
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09. Gaussian, Inc., Wallingford CT
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Hariharan PC, Pople JA (1973) The influence of polarization functions on molecular orbital hydrogenation energies. Theor Chim Acta 28:213–222
Francl MM, Pietro WJ, Hehre WJ, Binkley JS, Gordon MS, DeFrees DJ, Pople JA (1982) Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J Chem Phys 77:3654–3665
Hay PJ, Wadt WR (1985) Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J Chem Phys 82:270–283
Wadt WR, Hay PJ (1985) Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J Chem Phys 82:284–298
Lee SY, Bok KH, Kim C (2017) A fluorescence “turn-on” chemosensor for Hg2+ and ag+ based on NBD (7-nitrobenzo-2-oxa-1,3-diazolyl). RSC Adv 7:290–299
Vincenzo B, Cossi M (1998) Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J Phys Chem 102:1995–2001
Maurizio C, Vincenzo B (2001) Time-dependent density functional theory for molecules in liquid solutions. J Chem Phys 115:4708–4717
McNaught AD, Wilkinson A (1997) Limit of detection in analysis. IUPAC Compendium of Chemical Terminololy, 2nd edn. (the “Gold Book”) Blackwell Scientific Publications, Oxford
Benesi HA, Hildebrand JH (1949) A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J Am Chem Soc 71:2703–2707
Goswami S, Aich K, Das S, Das Mukhopadhyay C, Sarkar D, Mondal TK (2015) A new visible-light-excitable ICT-CHEF-mediated fluorescence ‘turn-on’ probe for the selective detection of Cd2+ in a mixed aqueous system with live-cell imaging. Dalton Trans 44:5763–5770
Acknowledgements
The National Research Foundation of Korea (NRF) (NRF-2018R1A2B6001686) is thankfully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 1037 kb)
Rights and permissions
About this article
Cite this article
Kim, C., Chae, J.B. A Highly Selective Fluorescent Chemosensor for Detecting Indium(III) with a Low Detection Limit and its Application. J Fluoresc 28, 1363–1370 (2018). https://doi.org/10.1007/s10895-018-2299-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-018-2299-z