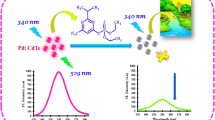
Abstract
A novel nanosensor based on CdSe quantum dots (QDs) capped with 8-hydroxyqunoline (HQ) was developed for Al3+ ions determination in aqueous solutions. The method is based on the fluorescence enhancement of the HQ functionalized QDs in the presence of Al3+ ions, due to the strong interaction between Al3+ and HQ. Prepared nanosensor exhibited an acceptable selectivity and sensitivity for Al3+ ions in the presence of other metal ions. Plot of Log(I/I0) against Log[Al3+] shows a good linearity in the range of 0.02–3.0 mM, and the method could be used for detection of Al3+ ions concentration in aqueous solutions.









Similar content being viewed by others
References
Verstraeten SV, Aimo L, Oteiza PI (2008) Aluminium and lead: molecular mechanisms of brain toxicity. Arch Toxicol 82(11):789–802
Delhaize E, Ryan PR (1995) Aluminum toxicity and tolerance in plants. Plant Physiol 107(2):315–321
Barcelo J, Poschenrieder C (2002) Fast root growth responses, root exudates, and internal detoxification as clues to the mechanisms of aluminium toxicity and resistance: a review. Environ Exp Bot 48(1):75–92
Proudfoot AT (2009) Aluminium and zinc phosphide poisoning. Clin Toxicol 47(2):89–100
Wang B, Xing W, Zhao Y, Deng X (2010) Effects of chronic aluminum exposure on memory through multiple signal transduction pathways. Environ Toxicol Pharmacol 29(3):308–313
Kunkel R, Manahan SE (1973) Atomic absorption analysis of strong heavy metal chelating agents in water and waste water. Anal Chem 45(8):1465–1468
Rezaaiyaan R, Hieftje G, Anderson H, Kaiser H, Meddings B (1982) Design and construction of a low-flow, low-power torch for inductively coupled plasma spectrometry. Appl Spectrosc 36(6):627–631
Bings NH, Bogaerts A, Broekaert JA (2006) Atomic spectroscopy. Anal Chem 78(12):3917–3946
Xu W, Zhou Y, Huang D, Su M, Wang K, Hong M (2014) A highly sensitive and selective fluorescent sensor for detection of Al3+ using a europium (III) quinolinecarboxylate. Inorg Chem 53(13):6497–6499
Sahana A, Banerjee A, Lohar S, Sarkar B, Mukhopadhyay SK, Das D (2013) Rhodamine-based fluorescent probe for Al3+ through time-dependent PET–CHEF–FRET processes and its cell staining application. Inorg Chem 52(7):3627–3633
Maity D, Govindaraju T (2010) Conformationally constrained (coumarin− triazolyl− bipyridyl) click fluoroionophore as a selective Al3+ sensor. Inorg Chem 49(16):7229–7231
Li Y-P, Liu X-M, Zhang Y-H, Chang Z (2013) A fluorescent and colorimetric sensor for Al 3+ based on a dibenzo-18-crown-6 derivative. Inorg Chem Commun 33:6–9
Li Y-W, Li J-R, Wang L-F, Zhou B-Y, Chen Q, Bu X-H (2013) Microporous metal–organic frameworks with open metal sites as sorbents for selective gas adsorption and fluorescence sensors for metal ions. J Mater Chem A 1(3):495–499
Ekimov A, Onushchenko A (1982) Quantum size effect in the optical-spectra of semiconductor micro-crystals. Soviet Physics Semiconductors-Ussr 16(7):775–778
Cai W, Shin D-W, Chen K, Gheysens O, Cao Q, Wang SX, Gambhir SS, Chen X (2006) Peptide-labeled near-infrared quantum dots for imaging tumor vasculature in living subjects. Nano Lett 6(4):669–676
Lee J, Govorov AO, Dulka J, Kotov NA (2004) Bioconjugates of CdTe nanowires and au nanoparticles: plasmon− exciton interactions, luminescence enhancement, and collective effects. Nano Lett 4(12):2323–2330
Eftekhari-Sis B, Malekan F, Younesi Araghi H (2018) CdSe quantum dots capped with p-nitrophenyldiazenylphenyloxadiazole: a nanosensor for Cd2+ ions in aqueous media. Can J Chem 96(4):371–376
Chen Y, Rosenzweig Z (2002) Luminescent CdS quantum dots as selective ion probes. Anal Chem 74(19):5132–5138
Lou Y, Zhao Y, Chen J, Zhu J-J (2014) Metal ions optical sensing by semiconductor quantum dots. J Mater Chem C 2(4):595–613
Eftekhari-Sis B, Karaminejad S, Malekan F, Araghi HY, Akbari A (2017) CdSe quantum dots based Nano-biosensor for detection of 185delAG mutation in BRCA1 gene, responsible for breast Cancer. J Inorg Organomet Polym Mater 27(6):1911–1917
Jiang X-h, B-d W, Z-y Y, Y-c L, Li T-r, Z-c L (2011) 8-Hydroxyquinoline-5-carbaldehyde Schiff-base as a highly selective and sensitive Al3+ sensor in weak acid aqueous medium. Inorg Chem Commun 14(8):1224–1227
Tang X-L, Peng X-H, Dou W, Mao J, Zheng J-R, Qin W-W, Liu W-S, Chang J, Yao X-J (2008) Design of a semirigid molecule as a selective fluorescent chemosensor for recognition of cd (II). Org Lett 10(17):3653–3656
Youk J-S, Kim YH, Kim E-J, Youn NJ, Chang S-K (2004) Hg2+-selective Chemosensor derived from 8-Hydroxyquinoline having Benzothiazole function in aqueous environment. Bulletin-Korean Chemical Society 25(6):869–872
Mukherjee M, Sen B, Pal S, Hundal MS, Mandal SK, Khuda-Bukhsh AR, Chattopadhyay P (2013) A cell permeable Cr 3+ selective chemosensor and its application in living cell imaging. RSC Adv 3(43):19978–19984
Qin J-c, Yang Z-y (2015) Selective fluorescent sensor for Al 3+ using a novel quinoline derivative in aqueous solution. Synth Met 209:570–576
Eftekhari-Sis B, Karaminejad S, Karimi F (2016) A Nano-biosensor for the detection of 185delAG mutation in BRCA1 gene, leading to breast Cancer. Cancer Investig 34(9):431–439
Eftekhari-Sis B, Mirdoraghi S (2016) Graphene oxide-terpyridine conjugate: a highly selective colorimetric and sensitive fluorescence Nano-chemosensor for Fe2+ in aqueous media. Nanochemistry Research 1(2):214–221
Audic N, Potier G, Sasaki NA (2013) New 2, 3-diaminopropionic acid inhibitors of AGE and ALE formation. Org Biomol Chem 11(5):773–780
Silva FO, Carvalho MS, Mendonça R, Macedo WA, Balzuweit K, Reiss P, Schiavon MA (2012) Effect of surface ligands on the optical properties of aqueous soluble CdTe quantum dots. Nanoscale Res Lett 7(1):536
Zhang H, Zhou Z, Yang B, Gao M (2003) The influence of carboxyl groups on the photoluminescence of mercaptocarboxylic acid-stabilized CdTe nanoparticles. J Phys Chem B 107(1):8–13
Oluwafemi S, Revaprasadu N, Ramirez A (2008) A novel one-pot route for the synthesis of water-soluble cadmium selenide nanoparticles. J Cryst Growth 310(13):3230–3234
Aldeek F, Mustin C, Balan L, Medjahdi G, Roques-Carmes T, Arnoux P, Schneider R (2011) Enhanced photostability from CdSe (S)/ZnO core/shell quantum dots and their use in biolabeling. Eur J Inorg Chem 2011(6):794–801
Cooper JK, Franco AM, Gul S, Corrado C, Zhang JZ (2011) Characterization of primary amine capped CdSe, ZnSe, and ZnS quantum dots by FT-IR: determination of surface bonding interaction and identification of selective desorption. Langmuir 27(13):8486–8493
Kosa SA, Al-Zhrani G, Salam MA (2012) Removal of heavy metals from aqueous solutions by multi-walled carbon nanotubes modified with 8-hydroxyquinoline. Chem Eng J 181:159–168
Zhang A, Bian Y, Wang J, Chen K, Dong C, Ren J (2016) Suppressed blinking behavior of CdSe/CdS QDs by polymer coating. Nano 8(9):5006–5014
Yu WW, Qu L, Guo W, Peng X (2003) Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals. Chem Mater 15(14):2854–2860
Wei G, Keller TF, Zhang J, Jandt KD (2011) Novel 1-D biophotonic nanohybrids: protein nanofibers meet quantum dots. Soft Matter 7(5):2011
Afaneh AT, Schreckenbach G (2015) Fluorescence enhancement/quenching based on metal orbital control: computational studies of a 6-thienyllumazine-based mercury sensor. J Phys Chem A 119(29):8106–8116
Acknowledgments
The work was supported by research council of the University of Maragheh. Iran Science Elites Federation (ISEF) was also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eftekhari-Sis, B., Samadneshan, K. & Vahdati-Khajeh, S. Design and Synthesis of Nanosensor Based on CdSe Quantum Dots Functionalized with 8-Hydroxyquinoline: a Fluorescent Sensor for Detection of Al3+ in Aqueous Solution. J Fluoresc 28, 767–774 (2018). https://doi.org/10.1007/s10895-018-2238-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-018-2238-z