Abstract
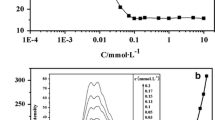
Micelles are of increasing importance as versatile carriers for hydrophobic substances and nanoprobes for a wide range of pharmaceutical, diagnostic, medical, and therapeutic applications. A key parameter indicating the formation and stability of micelles is the critical micelle concentration (CMC). In this respect, we determined the CMC of common anionic, cationic, and non-ionic surfactants fluorometrically using different fluorescent probes and fluorescence parameters for signal detection and compared the results with conductometric and surface tension measurements. Based upon these results, requirements, advantages, and pitfalls of each method are discussed. Our study underlines the versatility of fluorometric methods that do not impose specific requirements on surfactants and are especially suited for the quantification of very low CMC values. Conductivity and surface tension measurements yield smaller uncertainties particularly for high CMC values, yet are more time- and substance consuming and not suitable for every surfactant.










Similar content being viewed by others
References
Ashjari M, Khoee S, Mahdavian AR, Rahmatolahzadeh R (2012) Self-assembled nanomicelles using PLGA-PEG amphiphilic block copolymer for insulin delivery: a physicochemical investigation and determination of CMC values. J Mater Sci Mater Med 23(4):943–953. https://doi.org/10.1007/s10856-012-4562-1
Faustino CM, Calado AR, Garcia-Rio L (2009) New urea-based surfactants derived from alpha,omega-amino acids. J Phys Chem B 113(4):977–982. https://doi.org/10.1021/jp807396k
Garcia ME, Sanz-Medel A (1986) Dye-surfactant interactions: a review. Talanta 33(3):255–264. https://doi.org/10.1016/0039-9140(86)80060-1
Khan AM, Shah SS (2008) Determination of critical micelle concentration (Cmc) of sodium dodecyl sulfate (SDS) and the effect of low concentration of pyrene on its Cmc using ORIGIN software. J Chem Soc Pak 30(2):186–191
Song Z, Feng R, Sun M, Guo C, Gao Y, Li L, Zhai G (2011) Curcumin-loaded PLGA-PEG-PLGA triblock copolymeric micelles: preparation, pharmacokinetics and distribution in vivo. J Colloid Interface Sci 354(1):116–123. https://doi.org/10.1016/j.jcis.2010.10.024
Tadros TF (2005) Applied surfactants: principles and applications. Blackwell Science Publ, Oxford. https://doi.org/10.1002/3527604812
Mondal S, Ghosh S (2012) Role of curcumin on the determination of the critical micellar concentration by absorbance, fluorescence and fluorescence anisotropy techniques. J Photochem Photobiol B 115(0):9–15. https://doi.org/10.1016/j.jphotobiol.2012.06.004
Owen SC, Chan DPY, Shoichet MS (2012) Polymeric micelle stability. Nano Today 7(1):53–65. https://doi.org/10.1016/j.nantod.2012.01.002
Zhu Q, Huang L, Su J, Liu S (2014) A sensitive and visible fluorescence-turn-on probe for the CMC determination of ionic surfactants. Chem Commun 50(9):1107–1109. https://doi.org/10.1039/c3cc45244a
Anand U, Jash C, Mukherjee S (2011) Spectroscopic determination of critical micelle concentration in aqueous and non-aqueous media using a non-invasive method. J Colloid Interface Sci 364(2):400–406. https://doi.org/10.1016/j.jcis.2011.08.047
Nakahara Y, Kida T, Nakatsuji Y, Akashi M (2005) New fluorescence method for the determination of the critical micelle concentration by photosensitive monoazacryptand derivatives. Langmuir 21(15):6688–6695. https://doi.org/10.1021/la050206j
Prazeres TJV, Beija M, Fernandes FV, Marcelino PGA, Farinha JPS, Martinho JMG (2012) Determination of the critical micelle concentration of surfactants and amphiphilic block copolymers using coumarin 153. Inorg Chim Acta 381(0):181–187. https://doi.org/10.1016/j.ica.2011.09.013
Ghosh S, Krishnan A, Das PK, Ramakrishnan S (2003) Determination of critical micelle concentration by hyper-rayleigh scattering. J Am Chem Soc 125(6):1602–1606. https://doi.org/10.1021/ja029070r
Chiu YC, Kuo CY, Wang CW (2000) Using electrophoresis to determine zeta potential of micelles and critical micelle concentration. J Dispers Sci Technol 21(3):327–343. https://doi.org/10.1080/01932690008913270
Khamis M, Bulos B, Jumean F, Manassra A, Dakiky M (2005) Azo dyes interactions with surfactants. Determination of the critical micelle concentration from acid-base equilibrium. Dyes Pigments 66(3):179–183. https://doi.org/10.1016/j.dyepig.2004.09.012
Patist A, Bhagwat SS, Penfield KW, Aikens P, Shah DO (2000) On the measurement of critical micelle concentrations of pure and technical-grade nonionic surfactants. J Surfactant Deterg 3(1):53–58. https://doi.org/10.1007/s11743-000-0113-4
Staples E, Thompson L, Tucker I, Penfold J, Thomas RK, Lu JR (1993) Surface-composition of mixed surfactant monolayers at concentrations well in excess of the critical micelle concentration - a neutron-scattering study. Langmuir 9(7):1651–1656. https://doi.org/10.1021/La00031a009
Al-Soufi W, Pineiro L, Novo M (2012) A model for monomer and micellar concentrations in surfactant solutions: application to conductivity, NMR, diffusion, and surface tension data. J Colloid Interface Sci 370(1):102–110. https://doi.org/10.1016/j.jcis.2011.12.037
Aguiar J, Carpena P, Molina-Bolivar JA, Ruiz CC (2003) On the determination of the critical micelle concentration by the pyrene 1: 3 ratio method. J Colloid Interface Sci 258(1):116–122. https://doi.org/10.1016/S0021-9797(02)00082-6
Pérez-Rodríguez M, Prieto G, Rega C, Varela LM, Sarmiento F, Mosquera V (1998) A comparative study of the determination of the critical micelle concentration by conductivity and dielectric constant measurements. Langmuir 14(16):4422–4426. https://doi.org/10.1021/la980296a
Romani AP, Machado AE, Hioka N, Severino D, Baptista MS, Codognoto L, Rodrigues MR, de Oliveira HP (2009) Spectrofluorimetric determination of second critical micellar concentration of SDS and SDS/Brij 30 systems. J Fluoresc 19(2):327–332. https://doi.org/10.1007/s10895-008-0420-4
Chaudhuri R, Guharay J, Sengupta PK (1996) Fluorescence polarization anisotropy as a novel tool for the determination of critical micellar concentrations. J Photochem Photobiol A 101(2–3):241–244. https://doi.org/10.1016/S1010-6030(96)04412-7
Priev A, Zalipsky S, Cohen R, Barenholz Y (2002) Determination of critical micelle concentration of lipopolymers and other amphiphiles: comparison of sound velocity and fluorescent measurements. Langmuir 18(3):612–617. https://doi.org/10.1021/La0110085
Wong JE, Duchscherer TM, Pietraru G, Cramb DT (1999) Novel fluorescence spectral deconvolution method for determination of critical micelle concentrations using the fluorescence probe PRODAN. Langmuir 15(19):6181–6186. https://doi.org/10.1021/La981716z
Zhang X, Jackson JK, Burt HM (1996) Determination of surfactant critical micelle concentration by a novel fluorescence depolarization technique. J Biochem Biophys Methods 31(3–4):145–150. https://doi.org/10.1016/0165-022X(95)00032-M
Jumpertz T, Tschapek B, Infed N, Smits SH, Ernst R, Schmitt L (2011) High-throughput evaluation of the critical micelle concentration of detergents. Anal Biochem 408(1):64–70. https://doi.org/10.1016/j.ab.2010.09.011
Cai XT, Yang WJ, Huang L, Zhu QH, Liu SW (2015) A series of sensitive and visible fluorescence-turn-on probes for CMC of ionic surfactants: design, synthesis, structure influence on CMC and sensitivity, and fast detection via a plate reader and a UV light. Sensors Actuators B Chem 219:251–260. https://doi.org/10.1016/j.snb.2015.04.126
Kalyanasundaram K, Thomas JK (1977) Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. J Am Chem Soc 99(7):2039–2044. https://doi.org/10.1021/Ja00449a004
Chan DP, Owen SC, Shoichet MS (2013) Double click: dual functionalized polymeric micelles with antibodies and peptides. Bioconjug Chem 24(1):105–113. https://doi.org/10.1021/bc300511a
Cai L, Gochin M, Liu K (2011) A facile surfactant critical micelle concentration determination. Chem Commun 47(19):5527–5529. https://doi.org/10.1039/c1cc10605h
Chattopadhyay A, Harikumar KG (1996) Dependence of critical micelle concentration of a zwitterionic detergent on ionic strength: implications in receptor solubilization. FEBS Lett 391(1–2):199–202. https://doi.org/10.1016/0014-5793(96)00733-8
Lakowicz JR (2006) Principles of fluorescence spectroscopy 3. Auflage edn. Springer, Luxemburg
Resch-Genger U, Bremser W, Pfeifer D, Spieles M, Hoffmann A, DeRose PC, Zwinkels JC, Gauthier FO, Ebert B, Taubert RD, Monte C, Voigt J, Hollandt J, Macdonald R (2012) State-of-the art comparability of corrected emission spectra.1. Spectral correction with physical transfer standards and spectral fluorescence standards by expert laboratories. Anal Chem 84(9):3889–3898. https://doi.org/10.1021/ac2034503
Nakajima A (1976) Effects of isomeric solvents on vibronic band intensities in fluorescence-spectrum of pyrene. J Mol Spectrosc 61(3):467–469. https://doi.org/10.1016/0022-2852(76)90336-2
Regev O, Zana R (1999) Aggregation behavior of Tyloxapol, a nonionic surfactant Oligomer, in aqueous solution. J Colloid Interface Sci 210(1):8–17. https://doi.org/10.1006/jcis.1998.5776
Mukerjee P, Mysels KJ (1970) Critical micelle concentrations of aqueous surfactant systems. National Bureau of Standards, Washington, DC
Lianos P, Viriot ML, Zana R (1984) Study of the solubilization of aromatic-hydrocarbons by aqueous micellar solutions. J Phys Chem 88(6):1098–1101. https://doi.org/10.1021/J150650a014
Zana R, In M, Lévy H, Duportail G (1997) Alkanediyl-α,ω-bis(dimethylalkylammonium bromide). 7. Fluorescence probing studies of micelle micropolarity and microviscosity. Langmuir 13(21):5552–5557. https://doi.org/10.1021/la970369a
Jana B, Ghosh S, Chattopadhyay N (2013) Competitive binding of nile red between lipids and beta-cyclodextrin. J Photochem Photobiol B Biol 126:1–10. https://doi.org/10.1016/j.jphotobiol.2013.06.005
Sarkar N, Das K, Nath DN, Bhattacharyya K (1994) Twisted charge transfer processes of nile red in homogeneous solutions and in faujasite zeolite. Langmuir 10(1):326–329. https://doi.org/10.1021/la00013a048
Tajalli H, Gilani AG, Zakerhamidi MS, Tajalli P (2008) The photophysical properties of Nile red and Nile blue in ordered anisotropic media. Dyes Pigments 78(1):15–24. https://doi.org/10.1016/j.dyepig.2007.10.002
Felbeck T, Behnke T, Hoffmann K, Grabolle M, Lezhnina MM, Kynast UH, Resch-Genger U (2013) Nile-Red-nanoclay hybrids: red emissive optical probes for use in aqueous dispersion. Langmuir 29(36):11489–11497. https://doi.org/10.1021/la402165q
Chattopadhyay A, London E (1984) Fluorimetric determination of critical micelle concentration avoiding interference from detergent charge. Anal Biochem 139(2):408–412. https://doi.org/10.1016/0003-2697(84)90026-5
Danov KD, Kralchevsky PA, Ananthapadmanabhan KP (2014) Micelle-monomer equilibria in solutions of ionic surfactants and in ionic-nonionic mixtures: a generalized phase separation model. Adv Colloid Interface Sci 206(0):17–45. https://doi.org/10.1016/j.cis.2013.02.001
Garcia-Mateos I, Mercedes Velazquez M, Rodriguez LJ (1990) Critical micelle concentration determination in binary mixtures of ionic surfactants by deconvolution of conductivity/concentration curves. Langmuir 6(6):1078–1083. https://doi.org/10.1021/la00096a009
Williams RJ, Phillips JN, Mysels KJ (1955) The critical micelle concentration of sodium lauryl sulphate at 25 °C. Trans Faraday Soc 51(0):728. https://doi.org/10.1039/tf9555100728
Phillips JN (1955) The energetics of micelle formation. Trans Faraday Soc 51(4):561–569. https://doi.org/10.1039/Tf9555100561
Chiu YC, Wang SJ (1990) The micellar dissociation concentration of impure sodium dodecyl-sulfate systems in water. Colloids Surf 48(4):297–309. https://doi.org/10.1016/0166-6622(90)80236-W
Chiu YC, Lin YW (1996) A general method for determining the micellar dissociation concentration of a surfactant using a differential refractometer. Colloids Surf A 106(1):23–31. https://doi.org/10.1016/0927-7757(95)03341-6
Andreatta G, Bostrom N, Mullins OC (2005) High-Q ultrasonic determination of the critical nanoaggregate concentration of asphaltenes and the critical micelle concentration of standard surfactants. Langmuir 21(7):2728–2736. https://doi.org/10.1021/la048640t
Mehreteab A, Chen B (1995) Fluorescence technique for the determination of low critical micelle concentrations. J Am Oil Chem Soc 72(1):49–52. https://doi.org/10.1007/Bf02635778
Paillet S, Grassl B, Desbrieres J (2009) Rapid and quantitative determination of critical micelle concentration by automatic continuous mixing and static light scattering. Anal Chim Acta 636(2):236–241. https://doi.org/10.1016/j.aca.2009.02.011
Acknowledgements
We gratefully acknowledge the financial support from the Federal Ministry for Economic Affairs and Energy (Grant BMWi-10/12) and from the Ph.D. program of BAM. We are grateful to Mrs. G. Hidde for performing the surface tension measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scholz, N., Behnke, T. & Resch-Genger, U. Determination of the Critical Micelle Concentration of Neutral and Ionic Surfactants with Fluorometry, Conductometry, and Surface Tension—A Method Comparison. J Fluoresc 28, 465–476 (2018). https://doi.org/10.1007/s10895-018-2209-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-018-2209-4