Abstract
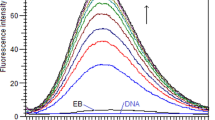
In the present work, the interaction of Isoxsuprine (ISX) with Calf thymus DNA (ct-DNA) under physiological conditions (Tris–HCl buffer of pH 7.4) was investigated by using electronic absorption, circular dichroism, viscosity, electrochemical studies, fluorescence techniques, salt effect studies and computational studies. Competitive fluorimetric studies with Hoechst 33258 have shown that ISX exhibit the ability to displace the DNA-bound Hoechst 33258, indicating that it binds to ct-DNA in strong competition with Hoechst 33258 for the minor groove binding. Furthermore, the resulting data showed that ISX cannot displace methylene blue or acridine orange, which are the common intercalator molecules. The viscosity of ct-DNA solution was almost unchanged on addition of ISX and circular dichroism (CD) spectra of ct-DNA showed small changes in the presence of ISX which is in agreement with groove binding mode of interaction. Thus all above studies showed that the ISX drug binds to ct-DNA in a groove binding mode.The salt-effect studies showed the non-electrostatic nature of binding of ISX to ct-DNA. Moreover, molecular docking results support the above experimental data and suggest that ISX prefers to bind on the minor groove of ct-DNA.














Similar content being viewed by others
References
Shahabadi N, Fili SM, Kheirdoosh F (2013) Study on the interaction of the drug mesalamine with calf thymus DNA using molecular docking and spectroscopic techniques. J Photochem Photobiol B Biol 128:20–26
Dogra S, Awasthi P, Nair M, Barthwal R (2013) Interaction of anticancer drug mitoxantrone with DNA hexamer sequence d-(CTCGAG)2 by absorption, fluorescence and circular dichroism spectroscopy. J Photochem Photobiol B Biol 123:48–54
Charak S, Mehrotra R (2013) Investigation of idarubicin–DNA interaction: Spectroscopic and molecular docking study. Int J Biol Macromol 60:213–218
Gua T, Hasebe Y (2012) Novel amperometric assay for drug–DNA interaction based on an inhibitory effect on an electrocatalytic activity of DNA–Cu(II) complex. Biosens Bioelectron 33:222–227
Williams AK, Dasilva SC, Bhatta A, Rawal B, Liu M, Korobkova EA (2012) Determination of the drug-DNA binding modes using fluorescence-based assays. Anal Biochem 422:66–73
Balakrishnan S, Jaldappagari S (2013) Binding of an anticancer Rutaceae plant flavonoid glycoside with calf thymus DNA: biophysical and electrochemical studies. J Lumin 142:17–22
Patel MN, Dosi PA, Bhatt BS (2012) Interaction of palladium(II) coordination compounds with calf thymus DNA and their antibacterial activity. Inorg Chem Commun 21:61–64
Singh MP, Joseph T, Kumar S, Bathini Y, Lown JW (1992) Synthesis and sequence-specific DNA binding of a topoisomerase inhibitory analog of Hoechst 33258 designed for altered base and sequence recognition. Chem Res Toxicol 5:597–607
Sparks J, Scholz C (2009) Evaluation of a cationic poly(β-hydroxyalkanoate) as a plasmid DNA delivery system. Biomacromolecules 10:1715–1719
Lown JW (1998) Anticancer Drug Des 3:25–40
Reynolds JEF (ed) (1996) Martindale: the extra pharmacopoeia, 31st edn. The Pharmaceutical Press, London
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38:3098–3100
Perdew JP (1986) Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys Rev B 33:8822–8824
Weigend F, Ahlrichs R (2005) Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Chem Phys 7:3297–3305
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JAJ, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill B, Johnson PMW, Chen W, Wong MW, Gonzalez C, Pople JA (2003) Gaussian software. Gaussian Inc, Pittsburgh
Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ (2007) Autodock version 4.0.1. The Scripps Research Institute, La Jolla
Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ (1998) Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem 19:1639–1662
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) Auto dock 4 and auto dock tools 4: automated docking with selective receptor flexibility. J Comput Chem 30:2785–2791
Wolfe A, Shimer GH, Meehan T (1987) Polycyclic aromatic hydrocarbons physically intercalate into duplex regions of denatured DNA. BioChemistry 26:6392–6396
Jalali F, Rasaee G (2015) Electrochemical, spectroscopic, and theoretical studies on the interaction between azathioprine and DNA. Int J Biol Macromol 81:427–434
Wu Y, Yang G (2010) Interaction between garcigenrin and DNA by spectrophotometry and fluorescence spectroscopy. Spectrosc Lett 43:28–35
Tao M, Zhang G, Pan J, Xiong C (2016) Deciphering the groove binding modes of tau-fluvalinate and flumethrin with calf thymus DNA. Spectrochim Acta A 155:28–37
Li JF, Dong C (2009) Study on the interaction of morphine chloride with deoxyribonucleic acid by fluorescence method. Spectrochim Acta A 71:1938–1943
Ross PD, Subramanian S (1981) Thermodynamics of protein association reactions: forces contributing to stability. BioChemistry 20:3096–3102
Yuan JL, Liu H, Kang X, Lv Z, Zou GL (2008) Characteristics of the isomeric flavonoids apigenin and genistein binding to hemoglobin by spectroscopic methods. J Mol Struct 891:333–339
Kashanian S, Ezzati Nazhad Dolatabadi J (2009) In vitro study of calf thymus DNA interaction with butylated hydroxyanisole. DNA Cell Biol 28:535–540
Zhang GW, Wang LH, Zhou XY, Li Y, Gong DM (2014) Binding characteristics of sodium saccharin with calf thymus DNA in vitro. J Agric Food Chem 62:991–1000
Kakkar R, Garg R (2002) Theoretical study of tautomeric structures and fluorescence spectra of Hoechst 33258. J Mol Struct THOECHEM 579:109–113
Guan Y, Zhou W, Yao X, Zhao M, Li Y (2006) Determination of nucleic acids based on the fluorescence quenching of Hoechst 33258 at pH 4.5. Anal Chim Acta 570:21–28
Rasaee G, Jalali F (2015) Electrochemical, spectroscopic, and theoretical studies on the interaction between azathioprine and DNA. Int J Biol Macromol 81:427–434
Bi S, Qiao C, Song D, Tian Y, Gao D, Sun Y, Zhang H (2006) Study of interactions of flavonoids with DNA using acridine orange as a fluorescence probe. Sensors Actuators B 119:199–208
Rodger A (1997) Circular dichroism and linear dichroism. Wiley Online Library
Ivanov V, Minchenkova L, Schyolkina A, Poletayev A (1973) Different conformations of double-stranded nucleic acid in solution as revealed by circular dichroism. Biopolymers 12:89–110
Lincoln P, Tuite E, Norden B (1997) Short-circuiting the molecular wire: cooperative binding of ∆-[Ru(phen)2dppz]2+ and ∆-[Rh(phi)2bipy]3+ to DNA. J Am Chem Soc 119:1454–1455
Rehman SU, Sarwar T, Husain MA, Ishqi HM, Tabish M (2015) Studying non-covalent drug–DNA interactions. Arch Biochem Biophys 576:49–60
Li FH, Zhao GH, Wu HX, Lin H, Wu XX, Zhu SR, Lin HK (2006) Synthesis, characterization and biological activity of lanthanum(III) complexes containing 2-methylene–1,10-phenanthroline units bridged by aliphatic diamines. J Inorg Biochem 100:36–43
Satyanarayana S, Dabrowiak JC, Chaires JB (1992) Neither ∆- nor ʌ-tris(phenanthroline)ruthenium(II) binds to DNA by classical intercalation. Biochemistry 31:9319–9324
Lincoln P, Norden B (1998) DNA binding geometries of ruthenium(II) complexes with 1,10-phenanthroline and 2,2′-bipyridine ligands studied with linear dichroism spectroscopy. Borderline cases of intercalation. J Phys Chem B 102:9583–9594
Bielawski K, Bielawska A, Anchim T, Wolczynski S (2005) Synthesis, DNA binding, topoisomerase inhibition and cytotoxic properties of 2-chloroethylnitrosourea derivatives of hoechst 33258. Biol Pharm Bull 28:1004–1009
Zhou X, Zhang G, Pan J (2015) Groove binding interaction between daphnetin and calf thymus DNA. Int J Biol Macromol 74:185–194
Fei Y, Lu G, Fan G, Wu Y (2009) Spectroscopic studies on the binding of a new quinolone antibacterial agent: sinafloxacin to DNA. Anal Sci 25:1333–1338
Bard AJ, Faulkner LR (2001) Electrochemical methods, second edn. Wiley, New York, p 36
Feng Q, Li NQ, Jiang YY (1997) Electrochemical studies of porphyrin interacting with DNA and determination of DNA. Anal Chim Acta 344:97–104
El-Sonbati AZ, Mohamed GG, El-Bindary AA, Hassan WMI, Diab MA, Morgan SM, Elkholy AK (2015) Supramolecular structure, molecular docking and thermal properties of azo dye complexes. J Mol Liq 212:487–502
Acknowledgements
The financial support from Bu-Ali Sina University Research Center is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Salehzadeh, S., Hajibabaei, F., Moghadam, N.H. et al. Binding Studies of Isoxsuprine Hydrochloride to Calf Thymus DNA Using Multispectroscopic and Molecular Docking Techniques. J Fluoresc 28, 195–206 (2018). https://doi.org/10.1007/s10895-017-2182-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-017-2182-3