Abstract
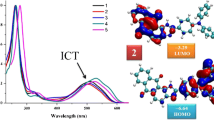
A series of novel unsymmetrically substituted indene-oxadiazole derivatives (3a–f) have been designed and synthesized by employing palladium catalysed Suzuki cross coupling reaction in high yields. The structural integrity of all the novel compounds was established by 1H, 13C NMR and LC/MS analysis. These compounds are amorphous in nature and are remarkably stable to long term storage under ambient conditions. The optoelectronic properties have been studied in detail using UV–Vis absorption and Fluorescence spectroscopy. All compounds emit intense blue to green-blue fluoroscence with high quantum yields. Time resolved measurments have shown life times in the range of 1.28 to 4.51 ns. The density functional theory (DFT) calculations were carried out for all the molecules to understand their structure–property relationships. Effect of concentration studies has been carried out in different concentrations for both absorption and emission properties and from this we have identified the optimized fluoroscence concentrations for all these compounds. The indene substituted anthracene-oxadiazole derivative (3f) showed significant red shift (λmax emi = 490 nm) and emits intense green-blue fluoroscence with largest stokes shift of 145 nm. This compound also exhibited highest fluoroscence life time (τ) of 4.51 ns, which is very close to the standard dye coumarin-540A (4.63 ns) and better than fluorescein-548 (4.10 ns). The results demonstrated that the novel unsymmetrical indene-substituted oxadiazole derivatives could play important role in organic optoelectronic applications, such as organic light-emitting diodes (OLEDs) or as models for investigating the fluorescent structure–property relationship of the indene-functionalized oxadiazole derivatives.








Similar content being viewed by others
References
Tannaci JF, Noji M, McBee JL, Tilley TD (2008) J Org Chem 73:7895
Kulkarni AP, Tonzola CJ, Babel A, Jenekhe SA (2004) Chem Mater 16:4556
Zaumseil J, Sirringhaus H (2007) Chem Rev 107:1296
Thomas SW, Joly GD, Swager TM (2007) Chem Rev 107:1339
Thompson BC, Fre’chet JMJ (2008) Angew Chem Int Ed 47:58
Mallesham G, Balaiah S, Ananth Reddy M, Sridhar B, Singh P, Srivastava R, Bhanuprakash K, Rao VJ (2014) Photochem Photobiol Sci 13:342
Zhu M, Yang C (2013) Chem Soc Rev 42:4963
Yoo S, Yun H, Kang I, Thangaraju K, Kwon S, Kim Y (2013) J Mater Chem C 1:2217
Nuyken O, Jungermann S, Wiederhirn V, Bacher E, Meerholz K (2006) Monatsh Chem 137:811
Kim YH, Lee SJ, Jung SY, Byeon KN, Kim JS, Shin SC, Kwon SK (2007) Bull Kor Chem Soc 28:443
Adhikari RM, Mondal R, Shah BK, Neckers DC (2007) J Org Chem 72:4727
Xu X, Yu G, Chen S, Di C, Liu Y (2008) J Mater Chem 18:299
Tong Q, Lai S, Chan M, Zhou Y, Kwong H, Lee C, Lee S (2008) Chem Mater 20:6310
Strukelj M, Papadimitrakopoulos F, Miller TM, Rothberg LJ (1995) Science 267:1969
Yoo B, Jones BA, Basu D, Fine D, Jung T, Mohapatra S, Facchetti A, Dimmler K, Wasielewski MR, Marks TJ, Dodabalapur A (2007) Adv Mater 19:4028
Katz HE, Lovinger AJ, Johnson J, Kloc C, Siegrist T, Li W, Lin YY, Dodabalapur A (2000) Nature 404:478
Tao Y, Yang C, Qin J (2011) Chem Soc Rev 40:2943
Zhu CC, Guo KP, Liu WB, He YB, Li ZM, Gao XC, Deng FJ, Wei B (2013) Opt Mater 35:2095
Okumoto K, Shirota Y (2001) Mater Sci Eng B 85:135
Kageyama H, Ohishi H, Tanaka M, Ohmori Y, Shirota Y (2010) IEEE J Quantum Electron 16:1528
Promarak V, Ichikawa M, Sudyoadsuk T, Saengsuwan S, Jungsuttiwong S, Keawin T (2008) Thin Solid Films 516:2881
Shirota Y (2000) J Mater Chem 10:1
Koene BE, Loy DE, Thompson ME (1998) Chem Mater 10:2235
Panchamukhi SI, Belavagi N, Rabinal MH, Khazi IA (2011) J Fluoresc 21:1515
Deshapande N, Belavagi NS, Panchamukhi SI, Rabinal MH, Khazi IAM (2014) Opt Mater 37:516
Dawson WR, Windsor MW (1968) J Phys Chem 72:3251
Wang C, Pålsson L-O, Batsanov AS, Bryce MR (2006) J Am Chem Soc 128:3789
Shailaja M, Anitha M, Manjula A, Rao BV (2010) Indian J Chem 49B:1088
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision A.1. Gaussian, Inc, Wallingford CT
Kamtekar KT, Wang C, Bettington S, Batsanov AS, Perepichka IF, Bryce MR, Ahn JH, Rabinal M, Petty MC (2006) J Mater Chem 16:3823
Prachumrak N, Pojanasopa S, Tarsang R, Namuangruk S, Jungsuttiwong S, Keawin T, Sudyoadsuk T, Promarak V (2014) New J Chem 38:3282
Collado D, Casado J, Gonzalez SR, Navarrete JTL, Suau R, Perez-Inestrosa E, Pappenfus TM, Raposo MMM (2011) Chem Eur J 17:498
Qu JQ, Pschirer NG, Liu DJ, Stefan A, Schryver FCD, Mullen K (2004) Chem Eur J 10:528
Ohkita H, Benten H, Anada A, Noguchi H, Kido N, Ito S, Yamamoto M (2004) Phys Chem Chem Phys 6:3977
Acknowledgments
We are thankful for the financial assistance from UGC, New Delhi under CPEPA and UPE-FAR-I programmes. NSB is thankful for the Research Fellowship under UPE FAR-I (F. No. 14-3/2012 (NS/PE), ND & GHP are thankful for the Research Fellowship under CPEPA (No. 8-2/2008(NS/PE). We greatly acknowledge University Science Instrumentation Centre for spectral analysis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 7206 kb)
Rights and permissions
About this article
Cite this article
Belavagi, N.S., Deshapande, N., Pujar, G.H. et al. Design, Synthesis and Optoelectronic Properties of Unsymmetrical Oxadiazole Based Indene Substituted Derivatives as Deep Blue Fluoroscent Materials. J Fluoresc 25, 1323–1330 (2015). https://doi.org/10.1007/s10895-015-1620-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-015-1620-3