Abstract
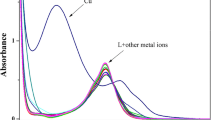
We report the selective recognition of sulfate anion in aqueous medium at biological pH 7.2 over the other interfering anions based on naphthoic acid bearing tripodal ligand by applying fluorescence turn off-on mechanism. The carboxylic acid groups in the ligand enhance the solubility in water and enable it to form complex with copper salt. Thus formed L-Cu2+ ensemble quench the fluorescence of the parent ligand and in turn recognize sulfate anion via revival of fluorescence intensity. The 1:2 stoichiometry was confirmed by ESI mass spectral data and Job’s plot. The average binding constant was found to be 6.2 × 108 M−2.

Tripodal receptor based on naphthoic acid forms complex with copper in water. This L-Cu2+ ensemble selectively recognize sulfate anion in aqueous medium at pH 7.2 over the other anions by fluorescence turn off-on mechanism





Similar content being viewed by others
References
Katayev EA, Ustynyuk YA, Sessler JL (2006) Coord Chem Rev 250:3004–3037
Weingärtner H, Franck EU, Weigand G, Dahmen N, Schwedt G, Frimmel FH, Gordalla BC, Johannsen K, Summers RS, Höll W, Jekel M, Gimbel R, Rautenbach R, Glaze HW (2000) Ullman’s Encyclopaedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. doi:10.1002/14356007.a28_001
Caltagirone C, Gale PA (2009) Chem Soc Rev 38:520–563
Gale PA (2006) Preface Coord Chem Rev 250:2917
Oton F, Tarraga A, Espinosa A, Velasco MD, Molina P (2006) J Org Chem 71:4590–4598
Jia C, Wu B, Li S, Huang X, Yang XJ (2010) Org Lett 12:5612–5615
Jia C, Wu B, Li S, Yang Z, Zhao Q, Liang J, Li QS, Yang XJ (2010) Chem Commun 46:5376–5378
Jia C, Wu B, Li S, Huang XJ, Zhao Q, Li QS, Yang XJ (2011) Angew Chem Int Ed 50:486–490
Prohens R, Martorell G, Ballester P, Costa A (2001) Chem Commun 16:1456–1457
Delgado-Pinar E, Rotger C, Costa A, Piña MN, Jiménez HR, Alarcón J, García-España E (2012) Chem Commun 48:2609–2611
Zeng Q, Cai P, Li Z, Qin J, Tang BZ (2008) Chem Commun 9:1094–1096
Li Z, Lou X, Yu H, Li Z, Qin J (2008) Macromolecules 41:7433–7439
Chung SY, Nam SW, Lim J, Park S, Yoon J (2009) Chem Commun 20:2866–2868
Zhang L, Lou X, Yu Y, Qin J, Li Z (2011) Macromolecules 44:5186–5193
Lou X, Mu H, Gong R, Fu E, Qin J, Li Z (2011) Analyst 136:684–687
Choi MG, Cha S, Lee H, Jeon HL, Chang SK (2009) Chem Commun 47:7390–7392
Cao X, Lin W, He L (2011) Org Lett 13:4716–4719
Lu W, Jiang H, Hu F, Jiang L, Shen Z (2011) Tetrahedron 67:7909–7912
Joseph R, Chinta JP, Rao CP (2010) Inorg Chim Acta 363:2833–2839
Wang H, Xue L, Jiang H (2011) Org Lett 13:3844–3847
Ma B, Zeng F, Zheng F, Wu S (2011) Chem Eur J 17:14844–14850
Hu ZQ, Wang XM, Feng YC, Ding L, Li M, Lin CS (2011) Chem Commun 47:1622–1624
Kar C, Das G (2013) J Photochem Photobiol A 251:128–133
Dey SK, Das G (2011) Chem Commun 47:4983–4985
Pramanik A, Das G (2009) Tetrahedron 65:2196–2200
Basu A, Das G (2011) Dalton Trans 40:2837–2843
Basu A, Das G (2012) Inorg Chem 51:1727–1738
Kar C, Basu A, Das G (2012) Tettrahedron Lett 53:4754–4757
Dutta BK, Kar C, Basu A, Das G (2012) Tettrahedron Lett 54:771–774
Kar C, Adhikari MD, Ramesh A, Das G (2013) Inorg Chem 52:742–752
Kar C, Adhikari MD, Ramesh A, Das G (2012) RSC Adv 2:9201–9206
Cikotiene I, Buksnaitiene R, Sazinas R (2011) Tetrahedron 67:706–717
Paeker A, Rees WT (1960) Analyst 85:587–600
Nguyen DM, Wang X, Ahn HY, Rodriguez L, Bondar MV, Belfield KD (2010) Appl Mater Interfaces 2:2978–2981
Neogi S, Bharadwaj PK (2006) Cryst Growth Des 6:433–438
Luan J, Sui FF, Lin HY, Lui GC, Xu C, Wang XL (2013) Cryst Growth Des 13:3561–3576
Chen Z, Gao DL, Diao CH, Liu Y, Ren J, Chen J, Zhao B, Shi W, Cheng P (2012) Cryst Growth Des 12:1201–1211
Liu GZ, Li SH, Wang LY (2012) CrystEngComm 14:880–889
Acknowledgments
G. D. gratefully acknowledges Council of Scientific and Industrial Research (01/2727/13/EMR-II) and Department of Science and Technology (DST), New Delhi, India, for financial support. NH and AB acknowledge Indian Institute of Technology Guwahati, India, for fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1.72 MB)
Rights and permissions
About this article
Cite this article
Hoque, M.N., Basu, A. & Das, G. Fluorescence Turn on Sensor for Sulfate Ion in Aqueous Medium Using Tripodal-Cu2+ Ensemble. J Fluoresc 24, 411–416 (2014). https://doi.org/10.1007/s10895-013-1306-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-013-1306-7