Abstract
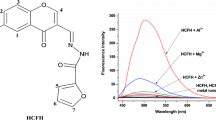
An oxadiazole derivative(OXD) containing symmetrical pyridine-2-formamidophenyl-binded moiety was synthesised as fluorescence turn-on sensor OA1. Its ultraviolet–visible(UV–vis) and fluorescent spectra(FS) gave prominent fluorescence enhancement only for monovalent silver ion(Ag+) in HEPES buffer solution (10 mM, pH = 7.0, DMF-H2O, 9:1, v/v), which indicated the photo-induced electron transfer(PET) occurred from the donor of pyridine-2-formamidophenyl group to oxadiazole fluorophore. The present study demonstrated that OA1 was a viable candidate as fluorescent receptor for a new Ag+ sensor. And the results of fluorescent spectral titration showed this sensor formed 1:1 complex with Ag+.






Similar content being viewed by others
References
Bissel RA, Silva AP, Gunaratne HQN, Lynch PLM, Maguire GEM, Sandanayake KRAS (1992) Chem Soc Rev 21:187–195
de Silva AP, Gunaratne HQN, Gunnlaugsson T, Huxley AJM, McCoy CP, Rademacher JT, Rice TE (1997) Chem Rev 97:1515–1566
Prodi L, Bolletta F, Montalti M, Zaccheroni N (2000) Coord Chem Rev 205:59–83
Valeur B, Leray I (2000) Coord Chem Rev 205:3–40
de Silva AP, Fox DB, Huxley AJM, Moody TS (2000) Coord Chem Rev 205:41–57
Resario M, Fabiola Z, Antonio C, Arturo E, Alberto T, Pedro M (2006) Org Lett 8:3235–3238
Tan H, Zhang Y, Chen Y (2011) Sensors Actuators B 156:120–125
Cheng X, Li S, Zhong A, Qin J, Li Z (2011) Sensors Actuators B 157:57–63
Peng X, Wang Y, Tang X, Liu W (2011) Dyes Pigments 91:26–32
Wang L, Yan JX, Qin W, Liu W, Wang R (2012) Dyes Pigments 92:1083–1090
Wang HH, Xue L, Yu CL, Qian YY, Jiang H (2011) Dyes Pigments 91:350–355
Zou Q, Jin J, Xu B, Ding L, Tian H (2011) Tetrahedron 67:915–920
Tang X, Liu H, Zou B, Tian D, Huang H (2012) Analyst 137:309–316
Lin LY, Chang LF, Jiang SJ (2008) J Agric Food Chem 56:6868–6872
Dolan SP, Nortrup DA, Bolger PM, Capar SG (2003) J Agric Food Chem 51:1307–1312
Filippelli M (1987) Anal Chem 59:116–123
Erxleben H, Ruzicka J (2005) Anal Chem 77:5124–5128
Yang H, Zhou Z, Huang K, Yu M, Li F, Yi T, Huang C (2007) Org Lett 9:4729–4737
Coskun A, Yilmaz MD, Akkaya EU (2007) Org Lett 9:607–616
Ko SK, Yang YK, Tae J, Shin I (2006) J Am Chem Soc 128:14150–14156
Zheng H, Qian ZH, Xu L, Yuan FF, Lan LD, Xu JG (2006) Org Lett 8:859–865
Matsushita M, Meijler MM, Wirsching P, Lerner RA, Janda KD (2005) Org Lett 7:4943–4949
Miller EW, Wong AP, Chang CJ (2006) J Am Chem Soc 128:9316–9323
Kwon JY, Jang YJ, Lee YJ, Kim KM, Seo MS, Nam W, Yoon J (2005) J Am Chem Soc 127:10107–10115
Weng YQ, Yue F, Zhong YR, Ye BH (2007) Inorg Chem 46:7749–7786
Zeng L, Miller EW, Pralle A, Isacoff EY, Chang CJ (2006) J Am Chem Soc 128:10–18
Royzen M, Dai Z, Canary JW (2005) J Am Chem Soc 127:1612–1618
Ando S, Koide K (2011) J Am Chem Soc 133:2556–2566
Song F, Watanabe S, Floreancig PE, Koide K (2008) J Am Chem Soc 130:16460–16468
Santra M, Roy B, Ahn KH (2011) Org Lett 13:3422–3427
Valeur B, Pouget J, Bouson J (1992) J Phys Chem C 96:6545–6552
Balzani V (2001) Electron transfer in chemistry. Wiley-VCH, Weinheim
Burdette SC, Walkup GK, Spingler B, Tsien RY, Lippard SJ (2001) J Am Chem Soc 123:7831–7837
Yoshida K, Mori T, Watanabe S, Kawai H, Nagamura T (1993) J Chem Soc Perkin Trans 2:393–398
Bissell RA, de Silva AP, Gunaratne HQN, Lynch MPL, Maguire GEM, Sandanayake KRAS (1992) Chem Soc Rev 21:187–195
Ren J, Wang QC, Qu DH, Zhao XL, Tian H (2004) Chem Lett 33:974–980
Masuhara H, Shioyama H, Saito T, Hamada K, Yasoshima S, Mataga N (1984) J Phys Chem 88:5868–5873
Zhang LJ, Zhou XQ, Li W (2003) Ind Eng Chem 20:481–488
Abdel MA (2006) J Chem Res 7:461–466
Klenov MS, Churakov AM, Anikin OV (2008) Russ Chem B+ 57:638–643
Jin QC, Anna L, Gavrilova B (2001) Inorg Chem 40:1386–1390
Li JB, Li NN, Yu XL (2010) J Wuhan Inst Tech 32:11–14
Acknowledgments
Financial support from General Program of National Natural Science Foundation of China (Grant No.51003047), Natural Science Foundation of Jiangsu Province (Grant No.55129003) and Higher Education Institutions Natural Science Foundation of Jiangsu Educational Commission (Grant No.09KJB540001, 10KJB540001) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zheng, C., Yuan, A., Zhang, Z. et al. Synthesis of Pyridine-Based 1,3,4-Oxadiazole Derivative as Fluorescence Turn-On Sensor for High Selectivity of Ag+ . J Fluoresc 23, 785–791 (2013). https://doi.org/10.1007/s10895-013-1213-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-013-1213-y