Abstract
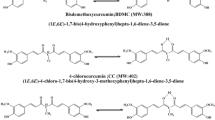
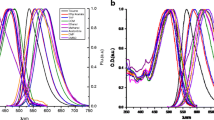
The steady-state absorption and fluorescence, as well as the time-resolved fluorescence properties of dimethoxycurcumin and bis-dehydroxycurcumin dissolved in several solvents differing in polarity and H-bonding capability are presented. The singlet oxygen generation efficiency of the two compounds relative to curcumin is estimated. The photodegradation quantum yield of the former compound in acetonitrile and methanol is determined. The dimethoxycurcumin and bis-dehydroxycurcumin decay mechanisms from the S 1 state are discussed and compared with those of curcumin, dicinnamoylmethane and bis-demethoxycurcumin.





Similar content being viewed by others
References
Mukhopadhyay A, Basu N, Ghatak N, Gujzal PK (1982) Anti-inflammatory and irritant activities of curcumin analogs in rats. Agents Actions 12:508–515
Srimal RC, Dhawan BN (1973) Pharmacology of di-ferulyl methane (curcumin), a non-steroidal anti-inflammatory agent. J Pharm Pharmacol 25:447–452
Rao T, Basu N, Ghatak N, Gujral PK (1982) Anti-inflammatory activity of curcumin analogs. Indian J Med Res 75:574–578
Khafif A, Schantz SP, Chou TC, Edelstein D, Sacks PG (1998) Quantification of chemopreventive synergism between epigallocatechin-3-gallate and curcumin in normal, premalignant and malignant human oral epithelial cells. Carcinogenesis 19:419–424
Leu TH, Maa MC (2002) The molecular mechanisms for the antitumorigenic effect of curcumin. Curr Med Chem 2:357–370
Woo J, Kim Y, Choi Y, Kim D, Lee K, Bae JH, Chang DS, Jeong YJ, Lee YH, Park J, Kwon TK (2003) Molecular mechanisms of curcumin-induced cyclotoxicity: induction of apoptosis through generation of reactive oxygen species, down-regulation of Bcl-XL and IAP, the release of cytochrome c and inhibition of Akt. Carcinogenesis 24:1199–1208
Moos PJ, Edes K, Mullally J, Fitzpatrick J (2004) Curcumin impairs tumor suppressor p53 function in colon cancer cells. Carcinogenesis 9:1611–1617
Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen P, Kayed R, Glabe CG, Frautschy SA, Cole GM (2004) Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem 280:5892–5901
Egan ME, Pearson M, Weiner SA, Rajendarn V, Rubin D, Glochner-Pagel J, Canney S, Du K, Lukacs GL, Caplan MF (2004) Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science 304:600–602
Aggrawal BB, Sundaram C, Malani N, Ichikawa H (2007) Curcumin: the Indian solid gold. Adv Exp Med Biol 595:1–75
Mazumder A, Neamati N, Sunder S, Schulz J, Perez H, Aich E, Pommier Y (1997) Curcumin analogs with altered potencies against HIV-1 integrase as probes for biochemical mechanisms of drug action. J Med Chem 40:3057–3063
Sui Z, Salto R, Li J, Craik C, Ortiz de Montellano PR (1993) Inhibition of the HIV-2 proteases by curcumin and curcumin boron complexes. Bioorg Med Chem 1:415–422
Bruzell E, Morisbak E, Tønnesen HH (2005) Studies on curcumin and curcuminoids. XXIX. Photoinduced cytotoxicity of curcumin in selected aqueous preparations. Photochem Photobiol Sci 4:523–530
Dahl TA, Bilski P, Reszka KJ, Chignell CF (1994) Photocytotoxicity of curcumin. Photochem Photobiol 59:290–294
Dahl TA, Mcgowan WM, Shand MA, Srinivasan VS (1989) Photokilling of bacteria by the natural dye curcumin. Arch Microbiol 151:183–185
Tonnesen HH, de Vries H, Karlsen J, van Henegouwen GB (1987) Studies on curcumin and curcuminoids IX: investigation of the photobiological activity of curcumin using bacterial indicator systems. J Pharm Sci 76:371–373
Haukvik T, Bruzell E, Kristensen S, Tønnesen HH (2009) Photokilling of bacteria by curcumin in different aqueous preparations. Studies on curcumin and curcuminoids. XXXVII. Pharmazie 64:666–673
Haukvik T, Bruzell E, Kristensen S, Tønnesen HH (2010) Photokilling of bacteria by curcumin in selected polyethylene glycol 400 (PEG 400) preparations. Studies on curcumin and curcuminoids XLI. Pharmazie 65:600–606
Haukvik T, Bruzell E, Kristensen S, Tønnesen HH (2011) A screening for antibacterial phototoxic effects of curcumin derivatives. Studies on curcumin and curcuminoids. XLIII. Pharmazie 66:69–74
Chignell CF, Bilski P, Reszka KJ, Motton AG, Sik RH, Dahl TA (1994) Spectral and photochemical properties of curcumin. Photochem Photobiol 59:295–302
Nardo L, Paderno R, Andreoni A, Haukvik T, Màsson M, Tønnesen HH (2008) Studies on curcumin and curcuminoids XXXII. Role of H-bond formation in the photoreactivity of curcumin. Spectroscopy 22:187–198
Nardo L, Andreoni A, Bondani M, Màsson M, Tønnesen HH (2009) Studies on curcumin and curcuminoids XXXIV. Photophysical properties of a symmetrical, non-substituted curcumin analogue. J Photochem Photobiol B: Biol 97:77–86
Nardo L, Andreoni A, Haukvik T, Màsson M, Tønnesen HH (2011) Studies on curcumin and curcuminoids XXXIX. Photophysical properties of bisdemethoxycurcumin. J Fluorescence 21:627–635
Gilli G, Bertolasi V (1990) In: Rappoport Z (ed) The chemistry of enols. Wiley, New York, pp 713–764
Tønnesen HH, Karlsen J, van Henegouwen GB (1986) Studies on curcumin and curcuminoids. VIII. Photochemical stability of curcumin. Z Lebensm Unters Forsch 183:116–122
Sundaryono A, Nourmamode A, Gardrat C, Grelier S, Bravic G, Chasseau D, Castellan A (2003) Studies on the photochemistry of 1,7-dipehnyl-1,6-heptadiene-3,5-dione, a non-phenolic curcuminoid model. Photochem Photobiol Sci 2:914–920
Emsley J (1984) In: Clarke MJ, Goodenough JB, Ibers JA, Jørgensen CK, Mingos DMP, Neilands JB, Reinen D, Sadler PJ, Weiss R, Williams RJP (eds) Structure and bonding. Springer Verlag, Berlin, pp 148–191
Strandjord AJG, Courtney SH, Friedrich DM, Barbara PF (1983) Excited-state dynamics of 3-hydroxyflavone. J Phys Chem 87:1125–1133
Weedon AC (1990) In: Rappoport Z (ed) The chemistry of enols. Wiley, New York, pp 591–638
Nikolov P, Fratev F, Petkov I, Markov P (1981) Dimer fluorescence of some β-dicarbonyl compounds. Chem Phys Lett 83:170–173
Tønnesen HH, Karlsen J, Mostad A (1982) Structural studies of curcuminoids. I. The crystal structure of curcumin. Acta Chem Scand B 36:475–479
Tonnesen HH, Karlsen J, Mostad A, Pedersen U, Rasmussen PB, Lawesson SO (1983) Structural studies of curcuminoids. II. Crystal structure of 1,7-Bis(4-hydroxyphenyl)-1,6-heptadiene-3,5-dione – Methanol complex. Acta Chem Scand B 37:179–185
Tonnesen HH, Karlsen J, Mostad A, Pedersen U, Rasmussen PB, Lawesson SO (1988) Structural studies of curcuminoids. IV. Crystal structure of 1,7-Bis(4-hydroxyphenyl)-1,6-heptadiene-3,5-dione Hydrate. Acta Chem Scand B 42:23–27
Mostad A, Pedersen U, Rasmussen PB, Lawesson SO (1983) Structural studies on curcuminoids. III. Crystal structure of 1,7-diphenyl-1,5-heptadiene-3,5-dione. Acta Chem Scand B 37:901–905
Gorbitz CH, Mostad A, Pedersen U, Rasmussen PB, Lawesson SO (1986) Structural studies on curcuminoids. V. Crystal structures of 1,7-bis(3,4-dimethoxyphenyl)-4-benzyl-1,6-heptadiene-3,5-dione (DDBHDD) and 1,7-bis(4-hydroxy-3-methoxy-phenyl)-4-(2-oxo-2-ethoxyethyl)-1,6-heptadiene-3,5-dione (DHMEDD). Acta Chem Scand B 40:420–429
Hansch C, Leo A, Taft RW (1991) A survey of Hammett substituent constants and resonance and field parameters. Chem Rev 91:165–195
Toullec J (1990) In: Rappoport Z (ed) The chemistry of enols. Wiley, New York, pp 324–398
Semenov SG, Khodyreva NV (1994) Theoretical study of electron-donor and spectroscopic properties pf substituted phenols in various solvents. J Mol Struct 337:89–97
Tomren MA, Màsson M, Loftsson T, Tønnesen HH (2007) Studies on curcumin and curcuminoids. XXXI. Symmetric and asymmetric curcuminoids: stability, activity and complexation with cyclodextrin. Int J Pharm 338:27–34
Velapoldi R, Tønnesen HH (2004) Corrected fluorescence spectra and quantum yields for a series of compounds in the visible spectral region. J Fluorescence 14:465–472
Nardo L, Bondani M, Andreoni A (2008) DNA-ligand binding mode discrimination by characterizing fluorescence resonance energy transfer through lifetime measurements with picosecond resolution. Photochem Photobiol 84:101–110
Moore DE (2004) In: Tønnesen HH (ed) Photostability of drugs and drug formulations. CRC, Boca Raton, pp 49–53
Kamlet MJ, Abboud JLM, Abraham MH, Taft RW (1983) Linear solvation energy relationships. 23. A comprehensive collection of the solvatochromic parameters, π*, α, and β, and some methods for simplifying the generalized solvatochromic equation. J Org Chem 48:2877–2887
Balasubramanian K (2006) Molecular orbital basis for yellow curry spice curcumin’s prevention of Alzheimer’s disease. J Agric Food Chem 54:3512–3520
Pedersen U, Rasmussen PB, Lawesson SO (1985) Synthesis of naturally occurring curcuminoids and related compounds. Liebigs Ann Chem 8:1557–1569
Ortica F, Rodgers MAJ (2001) A laser flash photolysis study of curcumin in dioxane-water mixtures. Photochem Photobiol 74:745–751
Galasso V, Kovac B, Modelli A, Ottaviani MF, Pichierri F (2008) Spectroscopic and theoretical study of the electronic structure of curcumin and related fragment molecules. J Phys Chem A 112:2331–2338
Wright JS (2002) Predicting the antioxidant activity of curcumin and curcuminoids. J Mol Struct (THEOCHEM) 591:207–217
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nardo, L., Andreoni, A., Bondani, M. et al. Studies on Curcumin and Curcuminoids. XLVI. Photophysical Properties of Dimethoxycurcumin and Bis-dehydroxycurcumin. J Fluoresc 22, 597–608 (2012). https://doi.org/10.1007/s10895-011-0995-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-011-0995-z