Abstract
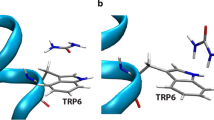
Urea and alkyl urea derivatives, which posses a free N-H moiety in the urea molecular framework is responsible for the fluorescence quenching of BSA. Fluorescence quenching accompanied with a blue initially and subsequently a red shift in the emission maximum of BSA is observed on the addition of urea derivatives containing N-H moieties. On the contrary, a fluorescence enhancement accompanied with a shift in the emission maximum towards the blue region is observed on the addition of tetramethylurea (TMU). Urea derivatives, which posses a free N-H moiety acts as a perfect denaturant by direct hydrogen-bonding interaction with BSA resulting in the unfolding process. The unfolding of the buried tryptophan moieties to the aqueous phase does not occur, when all the N-H moieties in the urea are methyl substituted (TMU). Fluorescence spectral techniques reveal that the direct hydrogen-bonding interaction of the N-H moiety of urea molecular framework with the carbonyl oxygen moieties of BSA results in the unfolding of the tryptophan moieties to the aqueous phase, while that of the carbonyl oxygen of urea with the N-H moieties of BSA is definitely not involved in the denaturation process. Steady state and time-resolved fluorescence studies illustrate that the extent of protein folding occurs at a relatively lower concentration of unsymmetrical alkyl urea derivatives (butyl urea (BU) and ethyl urea (EU)), compared to that of urea.








Similar content being viewed by others
References
Lakowicz JR (1999) Principles of fluorescence spectroscopy, 3rd edn. Kluwer Academic Plenum Publications, New York
Royer CA (2006) Probing protein folding and conformational transitions with fluorescence. Chem Rev 106:1769–1784
Hu YJ, Liu Y, Zhao RM, Dong JX, Qu SS (2006) Spectroscopic studies on the interaction between methylene blue and bovine serum albumin. J Photochem Photobiol 179:324–329
Steinhardt J, Krijn K, Leidy JG (1971) Differences between bovine and human serum albumins. Binding isotherms, optical rotatory dispersion, viscosity, hydrogen ion titration, and fluorescence effects. Biochemistry 10:4005–4015
Vladimirov JA (1965) Luminescence and photochemistry of proteins. Nauka, Moscow
Burstein EA, Vedenkina NS, Ivkova MN (1970) Fluorescence and the location of tryptophan residues in protein molecules. Photochem Photobiol 18:263–279
Carter DC, Chang B, Ho JX, Keeling K, Krishnasami Z (1994) Preliminary crystallographic studies of four crystal forms. Eur J Biochem 226:1049–1052
Brown JR, Shockley P (1982) In: Jost P, Griffith O (1 eds) Lipid-protein interactions, vol. 1. John Wiley & Sons, New York, pp 25–62
Carter DC, Ho JX (1994) Structure of serum albumins. Adv Protein Chem 45:153–202
Figgie J, Rossing TH, Fencl V (1991) The role of serum-proteins in acid-base equilibria. J Lab Clin Med 117:453–472
Kragh-Hansen U (1981) Molecular aspects of ligand binding of serum albumin. Pharmacol Rev 33:17–53
Curry S, Mandelkow H, Brick P, Franks N (1998) Crystal structure of human serum complexed with fatty acids reveals an asymmetric distributions of binding sites. Nat Sruct Biol 5:827–835
Hartley RW, Peterson EA, Sober HA (1962) The relaxation of free sulfydryl groups to chromatographic heterogeneity and polymerization of bovine plasma albumin. Biochemistry 1:60–68
Peterson HA, Foster JF (1965) The microheterogeneity of plasma albumins. II. Preparation and solubility properties of subfractions. J Biol Chem 240:2503–2507
Iqbal M, Veroll RE (1987) Volumetric properties of aqueous solutions of bovine serum albumin, and human hemoglobin. J Phys Chem 91:1935–1941
He XM, Carter DC (1992) Atomic structure and chemistry of human serum albumin. Nature 358:209–215
Peters T (1985) Serum albumins. Adv Protein Chem 37:161–245
Chadborn N, Byrant J, Barn AJ, O’Shea P (1999) Ligand-dependent confomational equilibria of serum albumin revealed by tryptophan fluorescence quenching. Biophys J 76:2198–2207
Dockal M, Carter DC, Ruker F (1999) The three recombinant domains of human serum albumin. structural characterization and ligand-binding properties. J Biol Chem 274:29303–29310
van der Spoel D, van Maaren PJ, Larsson P, Timneanu N (2006) Thermodynamics of hydrogen-bonding in hydrophillic and hydrophobic media. J Phys Chem B 110:4393–4398
Royer CA, Mann CJ, Matthews CR (1993) Resolution of the fluorescence equilibrium unfolding profile of trp aprorepressor using single tryptophan mutants. Prot Sci 2:1844–1852
Wang G, Lei Geng M (2006) Unfolding of apomyoglobin studied with two-dimensional correlations of tryptophan, 8-anilino-1-naphthalenesulfonate, and pyrene fluorescence. J Mol Struct 799:177–184
Vivian JT, Callis PR (2001) Mechanisms of tryptophan fluorescence shifts in proteins. Biophys J 80:2093–2109
Vanzi F, Madan B, Sharp K (1998) Effect of protein denaturants and guaniduim on water structure. A structural and thermodynamic study. J Am Chem Soc 120:10748–10753
Roumestand C, Boyer M, Guignard M, Barthe P, Royer CA (2001) Charecterization of the folding and unfolding reactions of a small β-barrel protein of novel topology the MTCP1 Oncogene product P13. J Mol Biol 312:247–259
Whitney PL, Tanford C (1962) Solubility of amino acids in aqueous urea solutions and its implications for the denaturation of proteins by urea. J Biol Chem 237:1735–1736
Nozaki Y, Tanford C (1963) The solubility of amino acids and related compounds in aqueous solutions. J Biol Chem 238:4074–4081
Tanford C (1968) Protein denauration. Adv Prot Chem 23:121–282
Tanford C (1969) Protein denauration, Theoretical models for the mechanism of denaturation. Adv Prot Chem 24:1–95
Simpson RB, Kauzmann W (1953) The kinetics of protein denaturation. I. The behavoiur of the optical rotation of ovalbumin in urea solutions. J Am Chem Soc 75:5167–5172
Katz S, Ferris TG (1966) Dialometric studies of the interaction of bovine serum albumin with Urea. Biochemistry 5:3246–3253
Katz S (1968) Partial molar volume and conformational changes produced by the denaturation of albumin by guanidine hydrochloride. Biochim Biophys Acta 154:468–477
Warren JR, Gordon JA (1971) Denaturation of globular proteins: III. A comparative study of the interaction of urea with several proteins. Biochim Biophys Acta 229:216–225
Gordon JA, Warren JR (1968) Denaturation of globular proteins. I. The interaction of urea and thiourea with bovine plasma albumin. J Biol Chem 243:5663–5669
Warren JR, Gordon JA (1970) Denaturation of globular proteins: II. The interaction of urea with lysozyme. J Biol Chem 245:4097–4104
Bonner OD (1978) Interaction of urea and substituted ureas with polyglycine and certain proteins. Physiol Chem Phys 10:25–35
Nandi PK, Robinson DR (1984) Effects of urea and guanidine hydrochloride on peptide and nonpolar groups. Biochemistry 23:6661–6668
Sulkwoska A (1999) Interaction between pyrimidine bases derivatives and serum albumin in the presence of urea. Colloids Surf A Physicochem Eng Asp 158:151–156
Zerovnik E, Lapanje S (1986) Interaction of myoglobin with urea and some alkylureas. Solvation in urea and alkylureas. Biophys Chem 24:53–59
Mayele M, Holz M, Sacco A (1999) NMR studies on hydrophobic interactions in solution. Phys Chem Chem Phys 1:4615–4618
Dotsch V, Wider G, Stegal G, Wuthrich K (1995) Interaction of urea with an unfolded protein The DNA-binding domain of the 434-repressor. FEBS Lett 366:6–10
Liepinsch E, Otting G (1994) Specificity of urea binding to proteins. J Am Chem Soc 116:9670–9674
Tirado-Rives J, Jorgensen WL (1991) Molecular dynamics simulations of the unfolding of an.alpha.-helical analog of ribonuclease A S-peptide in water. Biochemistry 30:3864–3871
Duffy EM, Kowalczyk PJ, Jorgensen WL (1993) Do denaturants interact with aromatic hydrocarbons. J Am Chem Soc 115:9271–9275
Jorgensen WL, Duffy EM, Tirado-Rives J (1993) Computational investigations of protein denaturation: apomyoglobin and chaotrope-arene interactions. Phil Trans Roy Soc London A 345:87–96
Thomas PD, Dill KA (1993) Local and non-local interactions in globular proteins and mechanism of alcohol denaturation. Prot Sci 2:2050–2065
Mountain R, Thirumalai D (2004) Importance of excluded volume on the solvation of Urea in water. J Phys Chem B 108:6826–6831
Kreschek GC, Scherega HA (1965) The temperature dependence of the enthalpy of formation of the amide hydrogen bonds. J Phys Chem 69:1704–1706
Schellman JA (1955) Thermodynamics of urea solutions and the heat of formation of the peptide hydrogen bond. Comput Rend Trav Lab Carlsberg CR Ser Chim 29:223–229
Stokes RH (1967) Thermodynamics of aqueous urea solutions. Aust J Chem 20:2067–2100
Kumaran R, Ramamurthy P (2006) PET supression of acridinedione dyes by urea derivatives in water and methanol. J Phys Chem B 110:23783–23789
Herskovits TT, Jaillet H, Gadegbeku B (1970) On the structural stability and solvent denaturation of proteins. J Biol Chem 245:4544–4550
Deep S, Ahluwalia J (2001) Interaction of bovine serum albumin with anionic surfactants. Phys Chem Chem Phys 3:4583–4591
Sulkwoska A, Bojko B, Rownicka J, Sulkwoski W (2003) Effect of urea on serum albumin complex with antithyroid drugs; fluorescence study. J Mol Struct 651–653:237–243
Itri R, Caetaro W, Barbosa LRS, Baptista MS (2004) Effect of urea on bovine serum albumin in aqueous and reverse micelle environments investigated by small angle X-ray scattering, fluorescence and circular dichroism. Braz J Phys 34:58–70
Swaminathan R, Krishnamoorthy G, Periasamy N (1994) Similarity of fluorescence lifetime distributions for single tryptophan proteins in random coil state. Biophys J 67:2013–2023
Kirby EP, Steiner RF (1970) Influence of solvent and temperature upon the fluorescence of indole derivatives. J Phys Chem 74:4480–4496
Ho C, Stubbs CD (1992) Hydration at the membrane protein-lipid interface. Biophys J 63:897–902
Krishna MMG, Periasamy N (1998) Fluorescence of organic dyes in lipid membranes: site of solubilisation on the viscosity and refractive index on lifetimes. J Fluoresc 8:81–91
Bartkowiak W, Zalesny R, Kowal M, Leszcynski J (2002) The influence of the solute/solvent interactions on the first-order hyperpolarizability in urea molecule. A quantum chemical study. Chem Phys Lett 362:224–228
Hoccart X, Turrell G (1999) Spectroscopic investigation of the dynamics of urea-water complexes by raman spectra. J Chem Phys 99:8498–8503
Lee C, Stahlberg EA, Fitzgerald G (1995) Chemical structure of urea in water. J Phys Chem 99:17737–17741
Grdadolnik J, Marechal Y (2002) Urea and urea–water solutions—an infrared study. J Mol Struc 615:177–189
Kuharski RA, Rossky PJ (1984) Molecular dynamics study of aqueous urea solution. J Am Chem Soc 106:5786–5793
Kuharski RA, Rossky PJ (1984) Solvation of hydrophobic species in aqueous urea solution, A molecular dynamics study. J Am Chem Soc 106:5794–6000
Tanaka H, Nakanishi K, Touhara H (1985) Computer experiments on aqueous solutions VII. Potential energy function for urea dimmer and molecular dynamics calculations of 8 mol% aqueous solution of urea. J Chem Phys 82:5184–5191
Masunov A, Dannenburg JJ (1999) Theoretical study of urea. I. Monomers and dimers. J Phys Chem A 103:178–184
Masunov A, Dannenburg JJ (2000) Theoretical study of urea and thiourea. 2. Chains and ribbons. J Phys Chem B 104:806–810
Rupprecht A, Kaatze U (2002) Solution properties of urea and its derivatives in water: evidence from ultrasonic relaxation spectra. J Phys Chem A 106:8850–8858
Mountain RD, Thirumalai D (2003) Molecular dynamics simulations of end-to-end contact formation in hydrocarbon chains in water and in aqueous urea solutions. J Am Chem Soc 125:1950–1954
Wallqvist A, Covell DG, Tirumalai D (1998) Hydrophobic interactions in aqueous urea solutions with implications for the mechanism of protein denaturation. J Am Chem Soc 120:427–428
Acknowledgements
Financial support by DST-IRHPA and UGC-INNOVATIVE Programme is acknowledged. R.K thanks the UGC for providing the financial assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.

S1
Absorption spectra of BSA as a function of [DMU]. (1) BSA alone, (2) BSA + DMU 1.0 M, (3) BSA + DMU 2.0 M, (4) BSA + DMU 4.0 M. (JPEG 63 kb)

S2
Absorption spectra of BSA as a function of [TMU]. (1) BSA alone, (2) BSA + TMU 0.2 M, (3) BSA + TMU 0.4 M, (4) BSA + TMU 0.8 M. (JPEG 66 kb)

S3
Absorption spectra of BSA as a function of [EU]. (1) BSA alone, (2) BSA + EU 0.5 M, (3) BSA + EU 1.0 M, (4) BSA + EU 2.0 M. (JPEG 64 kb)

S4
Absorption spectra of BSA as a function of [BU]. (1) BSA alone, (2) BSA + BU 0.5 M, (3) BSA + BU 1.0 M, (4) BSA + BU 2.0 M. (JPEG 64 kb)

S5
Emission spectra of BSA in the absence and presence of [DMU]. λex 295 nm. (1) 0.0 M, (2) 1.0 M, (3) 2.0 M, (4) 4.0 M, (5) 5.0 M, (6) 6.0 M, (7) 7.0 M, (8) 8.0 M. (JPEG 82 kb)

S6
3D Contour spectral studies of BSA with urea and symmetrical alkyl urea derivatives in aqueous solution. Excitation wavelength scan: 200–500 nm. Emission wavelength scan: 300–600 nm. a) BSA, b) BSA + Urea 1.2 M, c) BSA + DMU 1.2 M, d) BSA + TMU 1.2 M. (JPEG 171 kb)

S7
3D Contour spectral studies of BSA with unsymmetrical alkyl urea derivatives in aqueous solution. Excitation wavelength scan: 200–500 nm. Emission wavelength scan: 300–600 nm. a) BSA + MU 1.2 M, b) BSA + EU 1.2 M, c) BSA + BU 1.2 M. (JPEG 159 kb)

S8
Fluorescence decay of BSA in the absence and presence of [EU]. (1) Laser Profile, (2) 0.0 M, (3) 0.6 M, (4)1.2 M, (5) 2.4 M, (6) 4.8 M. (JPEG 79 kb)

S9
Fluorescence decay of BSA in the absence and presence of BU. (1) Laser Profile, (2) 0.0 M, (3) 0.6 M, (4)1.2 M, (5) 2.4 M,(6) 4.8 M. (JPEG 86 kb)
Rights and permissions
About this article
Cite this article
Kumaran, R., Ramamurthy, P. Denaturation Mechanism of BSA by Urea Derivatives: Evidence for Hydrogen-Bonding Mode from Fluorescence Tools. J Fluoresc 21, 1499–1508 (2011). https://doi.org/10.1007/s10895-011-0836-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-011-0836-0