Abstract
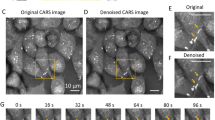
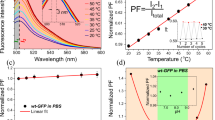
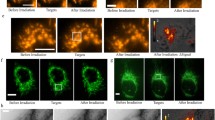
Various thermotherapies are based on the induction of lethal heat in target tissues. Spatial and temporal instabilities of elevated temperatures induced in therapy targets require optimized treatment protocols and reliable temperature control methods during thermotherapies. Heat-stress induced effects on mitochondrial transmembrane potentials were analyzed in breast cancer cells, species MX1, using the potential sensor JC-1 (Molecular Probes, Invitrogen, Germany). Potential dependant labeling of heat-stressed cells was imaged and evaluated by fluorescence microscopy and compared with control cells. JC-1 stains mitochondria in cells with high mitochondrial potentials by forming orange-red fluorescent J-aggregates while in cells with depolarized or damaged mitochondria the sensor dye exists as green fluorescent monomers. In MX1 cells orange-red and green fluorescence intensities were correlated with each other after various heat-stress treatments and states of mitochondrial membrane potentials were deduced from the image data. With increasing stress temperatures the intensity of red fluorescent J-aggregates decreased while the green fluorescence intensity of JC-1 monomers increased. This heat-stress response happened in a nonlinear manner with increasing temperatures resulting in a nonlinear increase of red/green fluorescence ratios. These data indicated that mitochondria in MX1 cells were increasingly depolarized in response to increasing ambient temperatures.




Similar content being viewed by others
References
Lindquist S (1986) The heat-shock response. Ann Rev Biochem. 55:1151–1191
Sonna LA, Fujita J, Gaffin SL, Lilly CM (2002) Effects of heat and cold stress on mammalian gene expression. J Appl Physiol 92(4):1725–1742
Takayama S, Reed JC, Homma S (2003) Heat-shock proteins as regulators of apoptosis. Oncogene 22(56):9041–9047
Kregel KC (2002) Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol 92(5):2177–2186
Gewiese B, Beuthan J, Fobbe F, Stiller D, Mueller G, Boese-Landgraf J, Wolf, K-J, Deimling M (1994) Magnetic resonance imaging-controlled laser-induced interstitial thermotherapy. Investig Radiol 29(3):345–351
Roggan A, Ritz J-P, Knappe V, Germer C- T, Isbert C, Schaedel D, Mueller G (2001) Radiation planning for thermal laser treatment. Med Laser Appl 16(2):65–72
Nikfarjam M, Christophi C (2003) Interstitial laser thermotherapy for liver tumours. Brit J Surg 90(9):1033–1047
Van Der Zee J (2002) Heating the patient: a promising approach? Ann Oncol 13(8):1173–1184
Debes A, Willers R, Goebel U, Wessalowski R (2004) Role of treatment in childhood cancers: Distinct resistance profiles of solid tumor cell lines towards combined thermochemotherapy. Pediatr Blood Cancer 45(5):663–669
Hehr T, Wust P, Bamberg M, Budach W (2003) Current potential role of thermoradiotherapy for solid tumors. Onkologie 26(3):295–302
Colombo R, Salonia A, Da Pozzo LF, Naspro R, Freschi M, Paroni R, Pavone Malasco M, Rigatti P (2003) Combination of intravesical chemotherapy and hyperthermia for the treatment of superficial bladder cancer: preliminary clinical experience. Crit Rev Oncol/Hematol 47(2):127–139
Jordan A, Scholz R, Maier-Hauff K, Johannsen M, Wust P, Nadobny J, Schirra H, Schmidt H, Deger S, Loening S, Lanksch W, Felix R (2001) Presentation of a new magnetic field therapy system fort he treatment of human solid tumors with magnetic fluid hyperthermia. J Magnetism Magn Mat 235:118–126
Johannsen M, Thiesen B, Gneveckow U, Taymoorian K, Waldoefner N, Scholz R, Deger S, Jung K, Loening S, Jordan A (2005) Thermotherapy using magnetic nanoparticles combined with external radiation in an orthotopic rat model of prostate cancer. Prostate 66(1):97–104
Gellermann J, Wlodarczyk W, Hildebrandt B, Ganter H, Nicolau A, Rau B, Tilly W, Faehling H, Nadobny J, Felix R, Wust P (2005) Noninvasive magnetic resonance thermotherapy of recurrent rectal carcinoma in a 1.5 Tesla hybrid system. Cancer Res 65(13):5872–5880
Mack M, Straub R, Eichler K, Söllner O, Lehnert T, Vogl T (2004) Breast cancer metastasis in liver: laser-induced interstitial thermotherapy-local tumor control rate and survival data. Radiol 233(2):400–409
Dewhirst MW, Viglianti BL, Lora-Michiels M, Hanson M, Hoopes PJ (2003) Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia 19(3):267–294
Park HG, Han SI, Oh SY, Kang HS (2005) Cellular responses to mild heat stress. Cell Mol Life Sci 62(1):10–23
Funk KRHW, Nagel F, Wanka F, Krinke, HE, Gölfert F, Hofer A (1999) Effects of heat shock on the functional morphology of cell organelles observed by video-enhanced microscopy. Anat Rec 255(4):458–464
Huckriede A, Heikema A, Sjollema K, Briones P, Agsteribbe E (1995) Morphology of the mitochondria in heat shock protein 60 deficient fibroblasts from mitochondrial myopathy patients. Effects of stress conditions. Virchows Arch 427(2):159–165
Lai YK, Lee WC, Hu CH, Hammond GL (1996) The mitochondria are recognition organelles of cell stress. J Surg Res 62(1):90–94
Macouillard-Poulletier de Gannes F, Leducq N, Diolez P, Belloc F, Merle M, Canioni P, Voison P-J (2000) Mitochondrial impairment and recovery after heat shock treatment in a human microglial cell line. Neurochem Intl 36(3):233–241
Macouillard-Poulletier de Gannes F, Merle M, Canioni P, Voison P-J (1998) Metabolic and cellular characterization of immortalized microglial cells under heat stress. Neurochem Intl 33(1):61–73
Waggoner AS (1979) Dye indicators of membrane potential. Annu Rev Biophys Bioenerg 8:47–68
Cossarizza A, Baccarani-Contri M, Kalashnikova G, Francheschi C (1993) A new method for the cytofluorimetric analysismof mitochondrial membrane potentialusing the J-aggregate forming lipophilic cation 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl- benzimidazolcarbocyanine iodide (JC-1). Biochem Biophys Res Commun 197(1):40–45
http://probes.invitrogen.com/servlets/spectra?fileid=3168p82
http://probes.invitrogen.com/media/publications/159.pdf
Keshavan P, Schwemberger SJ, Smith DLH, Babcock FG, Zucker SD (2004) Unconjugated bilirubin induces apoptosis in colon cancer cells by triggering mitochondrial depolarization. Int J Cancer 112(2):433–445
Zunino SJ, Storms DH (2005) Resveratrol-induced apoptosis is enhanced in acute lymphoblastic leukemia cells by modulation of the mitochondrial permeability transition pore. Cancer Lett XX:1–12
Lieven CJ, Vrabec JP, Levin LA (2003) The effects of oxidative stress on mitochondrial transmembrane potential in retinal ganglion cells. Antioxidants & Redox Signaling 5(5):641–646
Wang J-L, Ke D-S, Lin M-T (2005) Heat shock pretreatment may protect against heatstroke-induced circulatory shock and cerebral ischemia by reducing oxidative stress and energy depletion. Shock 23(2):161–167
Dressler C, Minet O, Novkov V, Mueller G, Beuthan J (2005) Microscopical heat stress investigations under application of quantum dots. J Biomed Optics 10:1–9
Beuthan J, Dressler C, Minet O (2004) Laser induced fluorescence detection of quantum dots redistributed in thermally stressed tumor cells. Laser Phys 14(2):213–19
Crompton M (1999) The mitochondrial permeability transition pore and its role in cell death. Biochem J 341(Pt 2):233–249
Kim J-S, He L, Lemasters JL (2003) Mitochondrial permeability transition: a common pathway to necrosis and apoptosis. Biochem Biophys Res Commun 304(3):463–470
Bernardi P, Scorrano L, Colonna R, Petronelli V, Di Lisa F (1999) Mitochondria and cell death. Eur J Biochem 264:687–701
O’Reilly CM, Fogarty KE, Drummond RM, Tuft RA, Walsh JV, Jr (2003) Quantitative analysis of spontaneous mitochondrial depolarizations. Biophys J 85:3350–3357
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dressler, C., Beuthan, J., Mueller, G. et al. Fluorescence Imaging of Heat-Stress Induced Mitochondrial Long-Term Depolarization in Breast Cancer Cells. J Fluoresc 16, 689–695 (2006). https://doi.org/10.1007/s10895-006-0110-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-006-0110-z