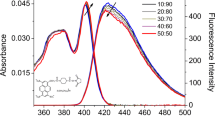
Abstract
The fluorescence lifetime strongly depends on the immediate environment of the fluorophore. Time-resolved fluorescence measurements of the enhanced forms of ECFP and EYFP in water–glycerol mixtures were performed to quantify the effects of the refractive index and viscosity on the fluorescence lifetimes of these proteins. The experimental data show for ECFP and EYFP two fluorescence lifetime components: one short lifetime of about 1 ns and a longer lifetime of about 3.7 ns of ECFP and for EYFP 3.4. The fluorescence of ECFP is very heterogeneous, which can be explained by the presence of two populations: a conformation (67% present) where the fluorophore is less quenched than in the other conformation (33% present). The fluorescence decay of EYFP is much more homogeneous and the amplitude of the short fluorescence lifetime is about 5%. The fluorescence anisotropy decays show that the rotational correlation time of both proteins scales with increasing viscosity of the solvent similarly as shown earlier for GFP. The rotational correlation times are identical for ECFP and EYFP, which can be expected since both proteins have the same shape and size. The only difference observed is the slightly lower initial anisotropy for ECFP as compared to the one of EYFP.
Similar content being viewed by others
References
R. Y. Tsien (1998). The green fluorescent protein. Annu. Rev. Biochem. 67, 509–544.
A. Miyawaki, A. Sawano, and T. Takako (2003). Lighting up cells: Labeling proteins with fluorophores. Suppl. Nature Cell Biol. 5, S1–S7.
M. A. Hink, N. V. Visser, J. W. Borst, A. van Hoek, and A. J. W. G. Visser (2003). Practical use of corrected fluorescence excitation and emission spectra of fluorescent proteins in Förster Resonance Energy Transfer (FRET) studies. J. Fluoresc. 13, 185–188.
K. Suhling, J. Siegel, D. Phillips, P. M. W. French, S. Lévêque-Fort, S. E. D. Webb, and D. M. Davis (2002). Imaging the environment of green fluorescent protein. Biophys. J. 83, 3589–3595.
S. J. Strickler and R. A. Berg (1962). Relationship between absorption intensity and fluorescence lifetime of molecules. J. Chem. Phys. 37, 814–820.
D. Toptygin, R. S. Savtchenko, N. D. Meadow, S. Roseman, and L. Brand (2002). Effect of solvent refractive index on the excited-state lifetime of a single tryptophan residue in a protein. J. Phys. Chem. B. 106, 3724–3734.
K. Suhling, D. M. Davis, and D. Phillips (2002). The influence of solvent viscosity on the fluorescence decay and time-resolved anisotropy of green fluorescent protein., J. Fluoresc. 12, 91–95.
A. Szabo (1984) Theory of fluorescence depolarization in macromolecules and membranes. J. Chem. Phys. 81, 150–167.
J. R. Lakowicz (1999). Principles of Fluorescence Spectroscopy, 2nd ed., Kluwer Academic/Plenum Publishers, New York.
M. A. Uskova, J. W. Borst, A. van Hoek, A. Schots, N. L. Klyachko, and A. J. W. G. Visser (2000). Fluorescence dynamics of green fluorescent proteins in AOT reversed micelles. Biophys. Chem. 87, 73–84.
A. Volkmer, V. Subramanian, D. J. S. Birch, and T. M. Jovin (2000). One- and two-photon excited fluorescence lifetimes and anisotropy decays of green fluorescent proteins. Biophys. J. 78, 1589–1598.
N. V. Visser, M. A. Hink, J. W. Borst, G. N. M. van der Krogt, and A. J. W. G. Visser (2002). Circular dichroism spectroscopy of fluorescent proteins. FEBS Lett. 521, 31–35.
K. Vos, A. van Hoek, and A. J. W. G. Visser (1987). Application of a reference deconvolution method to tryptophan fluorescence in proteins. A refined description of rotational dynamics. Eur. J. Biochem. 165, 55–63.
A. van Hoek and A. J. W. G. Visser (1985). Artefact and distortion sources in time correlated single photon counting. Anal. Instrum. 14, 359–378.
A. V. Digris, V. V. Skakun, E. G. Novikov, A. van Hoek, A. Claiborne, and A. J. W. G. Visser (1999). Thermal stability of a flavoprotein assessed from associative analysis of polarized time-resolved fluorescence spectroscopy. Eur. Biophys. J. 28, 526–531.
A. van Hoek, K. Vos, and A. J. W. G. Visser (1987). Ultrasensitive time-resolved polarized fluorescence spectroscopy as a tool in biology and medicine. IEEE J. Quant. Electron. QE-23, 1812–1820.
J. M. Beechem, E. Gratton, M. Ameloot, J. R. Knutson, and L. Brand (1991). The global analysis of fluorescence intensity and anisotropy decay data: Second generation theory and programs. in J. R. Lakowicz (Ed.), Topics in Fluorescence Spectroscopy, Plenum Press, New York, pp. 241–305.
M. Tramier, I. Gautier, T. Piolot, S. Ravalet, K. Kemnitz, J. Coppey, C. Durieux, V. Mignotte, and M. Coppey-Moisan (2002). Picosecond-hetero-FRET microscopy to probe protein–protein interactions in live cells. Biophys. J. 83, 3570–3577.
M. A. Rizzo, G. H. Springer, B. Granada, and D. W. Piston (2004). An improved cyan fluorescent protein variant useful for FRET. Nature Biotechnol. 22, 445–449.
S. Habuchi, M. Cotlet, J. Hofkens, G. Dirix, J. Michiels, J. Vanderleyden, V. Subramanian, and F. C. De Schryver (2002). Resonance energy transfer in a calcium concentration-dependent cameleon protein. Biophys. J. 83, 3499–3506.
J. Hyun Bae, M. Rubini, G. Jung, G. Wiegand, M. H. J. Seifert, M. K. Azim, J.-S. Kim, A. Zumbusch, T. A. Holak, L. Moroder, R. Huber, and N. Budisa (2003). Expansion of the genetic code enables design of a novel “gold” class of green fluorescent proteins. J. Mol. Biol. 328, 1071–1081.
J. W. Borst, M. A. Hink, A. van Hoek, and A. J. W. G. Visser (2003). Multiphoton microspectroscopy in living plant cells. Proc. SPIE 4963, 231–238.
F. S. Wouters, P. J. Verveer, and P. I. H. Bastiaens (2001). Imaging biochemistry inside cells. Trends Cell Biol. 11, 203–210.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Borst, J.W., Hink, M.A., van Hoek, A. et al. Effects of Refractive Index and Viscosity on Fluorescence and Anisotropy Decays of Enhanced Cyan and Yellow Fluorescent Proteins. J Fluoresc 15, 153–160 (2005). https://doi.org/10.1007/s10895-005-2523-5
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10895-005-2523-5