Abstract
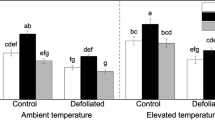
Although genetic, environmental, and G x E effects on aboveground phytochemistry have been well documented in trembling aspen (Populus tremuloides), little work has focused on the same factors affecting tissues underground. Belowground plant defenses are likely important mediators of root-feeding herbivores that can strongly influence plant fitness. We used a common garden of potted aspen trees to explore the individual and interactive effects of soil nutrient availability, foliar damage, genotype, and their interactions, on concentrations of phytochemicals in aspen roots. Our common garden experiment employed 12 aspen genotypes that were planted into either low- or high-nutrient soil environments. Half of the trees were subjected to defoliation for two successive years, while the others were protected from damage. At the end of the growing season after the second defoliation, we harvested the trees to obtain root samples for which we assessed levels of phenolic glycosides, condensed tannins, nitrogen, and starch. Phenolic glycosides were most affected by genotype, while the other root phytochemicals were most responsive to soil nutrient conditions. The effects of defoliation were observed in interaction with soil nutrient environment and/or genotype. Interestingly, the effect of defoliation on phenolic glycosides was mediated by soil nutrients, whereas the effect of defoliation on condensed tannins was observed in concert with effects of both soil nutrients and genotype. Comparison of data from this study with an earlier, related study revealed that concentrations of phenolic glycosides and condensed tannins are lower in roots than leaves, and less responsive to defoliation. That soil nutrient environment affects root phytochemical concentrations is not unexpected given the intimate association of roots and soil, but the complex interactions between soil nutrients, aboveground damage, and genotype, and their effects on root phytochemistry, are intriguing. Variation in root chemistry could have wide-reaching effects on soil microbial communities, nutrient cycling, and herbivores. Additionally, the response of phytochemicals to damage across organs can link different, spatially separated herbivores as they use different parts of the same plant resource.

Similar content being viewed by others
References
Andersen DC, MacMahon JA (1981) Population dynamics and bioenergetics of a fossorial herbivore, Thomomys talpoides (Rodentia: Geomyidae), in a spruce-fir sere. Ecol Monog 51:179–202
Ayres MP, Clausen TP, MacLean SF Jr, Redman AM, Reichardt PB (1997) Diversity of structure and antiherbivore activity in condensed tannins. Ecology 78:1696–1712
Bailey JK, Schweitzer JA, Rehill BJ, Lindroth RL, Martinsen GD, Whitham TG (2004) Beavers as molecular geneticists: a genetic basis to the foraging of an ecosystem engineer. Ecology 85:603–608
Bailey JK, Irschick DJ, Schweitzer JA, Rehill BJ, Lindroth RL, Whitham TG (2007) Selective herbivory by elk results in rapid shifts in the chemical composition of aspen forests. Biological Invasions 9:715–722
Barbehenn RV, Peter Constabel C (2011) Tannins in plant-herbivore interactions. Phytochemistry 72:1551–1565
Benson MK, Einspahr DW (1967) Early growth of diploid, triploid, and triploid hybrid aspen. Forest Sci 13:150–155
Boeckler GA, Gershenzon J, Unsicker SB (2011) Phenolic glycosides of the Salicaceae and their role as anti-herbivore defenses. Phytochemistry 72:1497–1509
Bryant JP, Chapin FS III, Klein DR (1983) Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 40:357–368
Cantor LF, Whitham TG (1989) Importance of belowground herbivory: pocket gophers may limit aspen to rock outcrop refugia. Ecology 70:962–970
Coyle DR, Mattson WJ, Raffa KF (2008) Invasive root-feeding insects in natural forest ecosystems of North America. In: Johnson SN, Murray JP (eds) Root feeders: An ecosystem perspective. CABI, Wallingford, pp 134–149
DeWoody J, Rowe CA, Hipkins VD, Mock KE (2008) “Pando” lives: molecular genetic evidence of a giant aspen clone in central Utah. West N Am Naturalist 68:493–497
Diner B, Berteaux D, Fyles J, Lindroth RL (2009) Behavioral archives link the chemistry and clonal structure of trembling aspen to the food choice of North American porcupine. Oecologia 160:687–695
Donaldson JR, Lindroth RL (2004) Cottonwood leaf beetle (Coleoptera: Chrysomelidae) performance in relation to variable phytochemistry in juvenile aspen (Populus tremuloides Michx.). Environ Entomol 33:1505–1511
Donaldson JR, Lindroth RL (2007) Genetics, environment, and their interaction determine efficacy of chemical defense in trembling aspen. Ecology 88:729–739
Erb M, Ton J, Degenhardt JR, Turlings TCJ (2008) Interactions between arthropod-induced aboveground and belowground defenses in plants. Plant Physiol 146:867–874
Falconer DS (1989) Introduction to quantitative genetics. Wiley, New York
Grant MC, Mitton JB, Linhart YB (1992) Even larger organisms. Nature 360:216
Gross HL, Syme PD (1981) Damage to aspen regeneration in northern Ontario by the ghost moth, Sthenopis quadriguttatus Grote. Can Forest Ser Res Notes 1:30–31
Hamilton JG, Zangerl AR, DeLucia EH, Berenbaum MR (2001) The carbon-nutrient balance hypothesis: its rise and fall. Ecol Lett 4:86–95
Havill NP, Raffa KF (1999) Effects of elicitation treatment and genotypic variation on induced resistance in Populus: impacts on gypsy moth (Lepidoptera: Lymantriidae) development and feeding behavior. Oecologia 120:295–303
Hendrick RL, Pregitzer KS (1992) The demography of fine roots in a northern hardwood forest. Ecology 73:1094–1104
Hinds TE (1985) Diseases. In: DeByle NV, Winokur RP (eds) Aspen: Ecology and management in western United States, Gen. Tech. Rep. RM-119. USDA Forest Service, Rocky Mountain Forest and Range Experiment Station, Fort Collins, CO, pp 87–106
Hol WHG, Macel M, Veen JA, Meijden E (2004) Root damage and aboveground herbivory change concentration and composition of pyrrolizidine alkaloids of Senecio jacobaea. Bas Appl Ecol 5:253–260
Holeski LM, Vogelzang A, Stanosz G, Lindroth RL (2009) Incidence of Venturia shoot blight in aspen (Populus tremuloides Michx.) varies with tree chemistry and genotype. Biochem Syst Ecol 37:139–145
Hwang S-Y, Lindroth RL (1997) Clonal variation in foliar chemistry of aspen: effects on gypsy moths and forest tent caterpillars. Oecologia 111:99–108
Hwang S-Y, Lindroth RL (1998) Consequences of clonal variation in aspen phytochemistry for late season folivores. Ecoscience 5:508–516
Johnson SN, Bezemer TM, Jones TH (2008) Linking aboveground and belowground herbivory. In: Johnson SN, Murray JP (eds) Root feeders: An ecosystem perspective. CABI, Wallingford, pp 53–170
Kainulainen P, Holopainen JK, Oksanen J (1995) Effects of SO2 on the concentrations of carbohydrates and secondary compounds in Scots pine (Pinus sylvestris L.) and Norway spruce (Picea abies (L.) Karst.) Seedlings. New Phytol 130:231–238
Kaplan I, Halitschke R, Kessler A, Sardanelli S, Denno RF (2008) Constitutive and induced defenses to herbivory in above- and belowground plant tissues. Ecology 89:392–406
Karban R, Baldwin IT (1997) Induced responses to herbivory. University of Chicago Press, Chicago
Keski-Saari S, Julkunen-Tiitto R (2003) Resource allocation in different parts of juvenile mountain birch plants: effect of nitrogen supply on seedling phenolics and growth. Physiol Plant 118:114–126
Kim J, Quaghebeur H, Felton GW (2011) Reiterative and interruptive signaling in induced plant resistance to chewing insects. Phytochemistry 72:1624–1634
Kosola KR, Dickmann DI, Paul EA, Parry D (2001) Repeated insect defoliation effects on growth, nitrogen acquisition, carbohydrates, and root demography of poplars. Oecologia 129:65–74
Kosola KR, Dickmann DI, Hall RB, Workmaster BA (2004) Cottonwood growth rate and fine root condensed tannin concentration. Tree Physiol 24:1063–1068
Kosola KR, Parry D, Workmaster BA (2006) Responses of condensed tannins in poplar roots to fertilization and gypsy moth defoliation. Tree Physiol 26:1607–1611
Kuikka K, Härmä E, Markkola A, Rautio P, Roitto M, Saikkonen K, Ahonen-Jonnarth U, Finlay R, Tuomi J (2003) Severe defoliation of Scots pine reduces reproductive investment by ectomycorrhizal symbionts. Ecology 84:2051–2061
Landhäusser SM, Lieffers VJ (2002) Leaf area renewal, root retention and carbohydrate reserves in a clonal tree species following above-ground disturbance. J Ecol 90:658–665
Landhäusser SM, Lieffers VJ (2003) Seasonal changes in carbohydrate reserves in mature northern Populus tremuloides clones. Trees 17:471–476
Lindroth RL, Hemming JDC (1990) Responses of the gypsy moth (Lepidoptera: Lymantriidae) to tremulacin, an aspen phenolic glycoside. Environ Entomol 19:842–847
Lindroth RL, Koss PA (1996) Preservation of Salicaceae leaves for phytochemical analyses: further assessment. J Chem Ecol 22:765–771
Lindroth RL, Peterson SS (1988) Effects of plant phenols on performance of southern armyworm larvae. Oecologia 75:185–189
Lindroth RL, St. Clair SB (2013) Adaptations of quaking aspen (Populus tremuloides Michx.) for defense against herbivores. Forest Ecol Manag 299:14–21
Lindroth RL, Kinney KK, Platz CL (1993) Responses of deciduous trees to elevated atmospheric CO2: productivity, phytochemistry and insect performance. Ecology 74:763–777
Lindroth RL, Osier TL, Barnhill HRH, Wood SA (2002) Effects of genotype and nutrient availability on phytochemistry of trembling aspen (Populus tremuloides Michx.) during leaf senescence. Biochem Syst Ecol 30:297–307
Lindroth RL, Donaldson JR, Stevens MT, Gusse AC (2007) Browsing quality in quaking aspen (Populus tremuloides): effects of genotype, nutrients, defoliation, and coppicing. J Chem Ecol 33:1049–1064
Madritch MD, Lindroth RL (2011) Soil microbial communities adapt to genetic variation in leaf litter inputs. Oikos 120:1696–1704
Mattson WJ, Herms DA, Witter JA, Allen DC (1991) Woody plant grazing systems: North American outbreak folivores and their host plants. In: Baranchikov YN, Mattson WJ, Hain FP, Payne TL (eds) Forest insect guilds: patterns of interaction with host trees, Gen. Tech. Rep. NE-153. USDA Forest Service, Northeastern Forest Experiment Station, pp 53–84
Mattson WJ, Julkunen-Tiitto R, Herms DA (2005) CO2 enrichment and carbon partitioning to phenolics: do plant responses accord better with the protein competition or the growth differentiation balance models? Oikos 111:337–347
McKey D (1979) The distribution of secondary compounds within plants. In: Rosenthal GA, Janzen DH (eds) Herbivores: Their interactions with secondary plant metabolites. Academic Press, New York, pp 55–133
Muller RN, Kalisz PJ, Luken JO (1989) Fine root production of astringent phenolics. Oecologia 79:563–565
Nord JC, Knight FB, Vogt GB (1965) Identity and biology of an aspen root girdler, Agrilus horni. Forest Sci 11:33–41
Osier TL, Lindroth RL (2001) Effects of genotype, nutrient availability, and defoliation on aspen phytochemistry and insect performance. J Chem Ecol 27:1289–1313
Osier TL, Lindroth RL (2004) Long-term effects of defoliation on quaking aspen in relation to genotype and nutrient availability: plant growth, phytochemistry and insect performance. Oecologia 139:55–65
Parry D, Herms DA, Mattson WJ (2003) Responses of an insect folivore and its parasitoids to multiyear experimental defoliation of aspen. Ecology 84:1768–1783
Porter LJ, Hrstich LN, Chan BG (1986) The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 25:223–230
Radwan MA, Crouch GL, Harrington CA, Ellis WD (1982) Terpenes of ponderosa pine and feeding preferences by pocket gophers. J Chem Ecol 8:241–253
Rasmann S, Agrawal AA (2008) In defense of roots: a research agenda for studying plant resistance to belowground herbivory. Plant Physiol 146:875–880
Rhoades DF (1979) Evolution of plant chemical defense against herbivores. In: Rosenthal GA, Janzen DH (eds) Herbivores: Their interactions with secondary plant metabolites. Academic Press, New York, pp 3–54
SAS Institute Inc (2012) JMP Version 10.0.2. SAS Institute Inc, Cary
Schier GA, Zasada JC (1973) Role of carbohydrate reserves in the development of root suckers in Populus tremuloides. Can J Forest Res 3:243–250
Seastedt TR, Ramundo RA, Hayes DC (1988) Maximization of densities of soil animals by foliage herbivory: empirical evidence, graphical and conceptual models. Oikos 51:243–248
Siemens DH, Lischke H, Maggiulli N, Schurch S, Roy BA (2003) Cost of resistance and tolerance under competition: the defense-stress benefit hypothesis. Evol Ecol 17:247–263
Soler R, Bezemer TM, Cortesero AM, Van der Putten WH, Vet LEM, Harvey JA (2007) Impact of foliar herbivory on the development of a root-feeding insect and its parasitoid. Oecologia 152:257–264
Stevens MT, Lindroth RL (2005) Induced resistance in the indeterminate growth of aspen (Populus tremuloides). Oecologia 145:298–306
Stevens MT, Waller DM, Lindroth RL (2007) Resistance and tolerance in Populus tremuloides: genetic variation, costs, and environmental dependency. Evol Ecol 21:829–847
Stevens MT, Kruger EL, Lindroth RL (2008) Variation in tolerance to herbivory is mediated by differences in biomass allocation in aspen. Funct Ecol 22:40–47
Stevens MT, Gusse AC, Lindroth RL (2012) Genotypic differences and prior defoliation affect re-growth and phytochemistry after coppicing in Populus tremuloides. J Chem Ecol 38:306–314
Stowe KA, Marquis RJ, Hochwender CG, Simms EL (2000) The evolutionary ecology of tolerance to consumer damage. Annu Rev Ecol Syst 31:565–595
van Dam NM (2009) Belowground herbivory and plant defenses. Annu Rev Ecol Syst 40:373–391
van Dam NM, Tytgat T, Kirkegaard J (2009) Root and shoot glucosinolates: a comparison of their diversity, function and interactions in natural and managed ecosystems. Phytochem Rev 8:171–186
Ward AL, Keith JO (1962) Feeding habits of pocket gophers on mountain grasslands, Black Mesa, Colorado. Ecology 43:744–749
Whittaker JB (2003) Root–animal interaction. In: De Kroon H, Wisser EJW (eds) Root ecology. Springer-Verlag, Berlin Heidelberg, New York, pp 363–385
Wooley SC, Walker S, Vernon J, Lindroth RL (2008) Aspen decline, aspen chemistry, and elk herbivory: are they linked? Rangelands 30:17–21
Wright JW (1976) Introduction to forest genetics. Academic Press, New York
Zangerl AR, Bazzaz FA (1992) Theory and pattern in plant defense allocation. In: Fritz RS, Simms EL (eds) Plant resistance to herbivores and pathogens: ecology, evolution, and genetics. University of Chicago Press, Chicago, pp 363–391
Acknowledgments
We thank Stuart Wooley for encouraging us to conduct this work, and the many undergraduate assistants who helped harvest roots, especially Helen Bothwell, Laura Mortimore, and Laura Riel. Comments from two anonymous reviewers helped improve the manuscript. This research was funded by National Science Foundation grants DEB-0074424 and DEB-0841609 to RLL. MTS was supported by a STAR (Science To Achieve Results) Fellowship from the Environmental Protection Agency.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stevens, M.T., Gusse, A.C. & Lindroth, R.L. Root Chemistry in Populus tremuloides: Effects of Soil Nutrients, Defoliation, and Genotype. J Chem Ecol 40, 31–38 (2014). https://doi.org/10.1007/s10886-013-0371-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-013-0371-3