Abstract
The pine weevil Hylobius abietis (L.) is a severe pest of conifer seedlings in reforested areas of Europe and Asia. To identify minimally toxic and ecologically sustainable compounds for protecting newly planted seedlings, we evaluated the volatile metabolites produced by microbes isolated from H. abietis feces and frass. Female weevils deposit feces and chew bark at oviposition sites, presumably thus protecting eggs from feeding conspecifics. We hypothesize that microbes present in feces/frass are responsible for producing compounds that deter weevils. Here, we describe the isolation of a fungus from feces and frass of H. abietis and the biological activity of its volatile metabolites. The fungus was identified by morphological and molecular methods as Penicillium expansum Link ex. Thom. It was cultured on sterilized H. abietis frass medium in glass flasks, and volatiles were collected by SPME and analyzed by GC-MS. The major volatiles of the fungus were styrene and 3-methylanisole. The nutrient conditions for maximum production of styrene and 3-methylanisole were examined. Large quantities of styrene were produced when the fungus was cultured on grated pine bark with yeast extract. In a multi-choice arena test, styrene significantly reduced male and female pine weevils’ attraction to cut pieces of Scots pine twigs, whereas 3-methylanisole only reduced male weevil attraction to pine twigs. These studies suggest that metabolites produced by microbes may be useful as compounds for controlling insects, and could serve as sustainable alternatives to synthetic insecticides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adult pine weevils, Hylobius abietis (L.), feed on the tender bark of a wide range of conifers. Small plants are the preferred host in reforested areas of managed forests (Day et al., 2004), and weevils can kill up to 80 % of planted pine and spruce seedlings every year if no preventive measures are taken (Petersson and Örlander, 2003). Seedlings are protected routinely by insecticide treatment before planting, but insecticides may contaminate soil and water and may thus have significant adverse effects on the environment (Peshin et al., 2009). Non-targeted organisms also may be affected, leading to ecological disturbance. In addition, there is a concern that insecticide-treated seedlings may affect the health of forestry workers (Kolmodin-Hedman et al., 1995). It is necessary to develop alternative methods of controlling pest insects using naturally occurring and sustainable insect repellents and anti-feedants. At present, plants are the major source of such chemicals, but microbes including fungi and bacteria also produce a variety of insect repellents (Daisy et al., 2002; Dong et al., 2007; Sun et al., 2008; Lam et al., 2010) such as avermectin, a macrocyclic lactone isolated from a bacterial fermentation broth that has been successfully used for pest control (Hotson, 1982).
We are interested in finding sustainable and ecologically non-disruptive methods to limit pine weevil damage on newly planted seedlings. Observations of oviposition and egg-protecting behavior of pine weevil females reveal that they lay their eggs in the roots of fresh conifer stumps. A cavity is gnawed in the bark and an egg is deposited in the cavity. After this, the female adds her feces and closes the cavity using fresh pieces of chewed bark. It has been suggested that the deposition of feces protects the eggs from conspecific feeding (Borg-Karlson et al., 2006). Although the function of added feces is not fully understood, it has been shown that compounds present in feces (e.g., methylanisole, p-cresol, and dihydroconiferylalcohol) have an anti-feedant effect on pine weevils (Borg-Karlson et al., 2006). Structure–activity studies have identified a large number of potent antifeedants, mainly derivatives of benzoic acid methyl esters, and various phenylpropanoids and phenylpropenyl esters (Legrand et al., 2004; Unelius et al., 2006; Sunnerheim et al., 2007; Bohman et al., 2008).
Our hypothesis is that compounds responsible for anti-feedant and repellent effects on pine weevils originate from microbes present in frass and feces of ovipositing females. These microbes grow in the humid environment around the eggs and produce compounds that repel weevils continuously. A similar observation also has been made by Lam et al. in 2010: the volatiles produced by a fungus growing on chicken feces were found to repel domestic house files and decrease their oviposition rate. Identification of insect-controlling chemicals produced by microbes and a clear understanding of how these natural products function may lead to the development of alternatives to insecticides.
We describe the isolation and identification of the fungus Penicillium expansum Link ex. Thom associated with pine weevil frass and feces. The fungus was cultured on its natural medium (i.e., pine weevil frass) and volatiles released by the fungus were collected by SPME and analyzed using GC-MS. Various culture media were used to compare the production of the major fungus volatiles, which were identified as styrene and 3-methylanisole. Styrene and 3-methylanisole were tested for biological activity against pine weevil using a multi-choice laboratory bioassay.
Methods and Materials
Chemicals
(+)-α-pinene, (+)-3-carene, (+)-β-pinene, (+)-limonene, octanal, styrene, and 3-methylanisole (all 99 % GC-purity), dimethyl sulfoxide, potato dextrose agar, glucose and yeast extract were purchased from Sigma-Aldrich, Sweden; agar was purchased from Fisher Scientific, Sweden.
Collection of Pine Weevil Frass and Feces
Pine weevils (H. abietis) of both sexes were collected in the field and starved for 24 h; water was provided by placing a sterilized wet filter paper in the container. Two hundred starved weevils, including both sexes, were placed for 2 d in a sterilized container with a sieve at the bottom, which fit into another sterilized container. The weevils were provided with fresh Pinus sylvestris twigs obtained from a single healthy tree. The15-30 mm diam, 100 mm long twigs were surface sterilized in 70 % ethanol, and excessive ethanol was evaporated aseptically before feeding them to weevils. The pine weevil frass (mixture of feces and pine bark particles chewed by weevils) fell through the sieve into the container below for aseptic collection and storage at 5 °C in a sterilized glass vial.
Fully fed weevils were placed in sterilized and dried glass Petri plates, where they were kept for 24 h. After removing the weevils, their feces were collected with the aid of a razor blade and aseptically transferred to a glass vial for storage at 5 °C. Frass and feces collection was repeated three times using fresh P. sylvestris twigs that were picked on different dates in the autumn from three self-regenerated, healthy trees that were about 10 y-old, located in the Lunsen forest south of Uppsala, Sweden (59o 46′N, 17o 40′E).
Isolation of Fungus from Pine Weevil Frass
We prepared pine weevil frass agar (WFA) in 90 mm Petri plates using 200 mg weevil frass, 300 mg agar, and 20 ml distilled water, autoclaved for 45 min at 121 °C and 20 psi. Pure fungal strains were isolated according to methods described previously (Peterson et al., 2009; Lam et al., 2010). Aseptically collected pine weevil frass (5 g) was wet with 25 ml sterilized water in a 100 ml glass beaker and incubated at room temperature (22 °C ± 2); two replicates were prepared at a time. The surface of the frass was inspected daily over a period of 25 d. Newly emerging fungus was inoculated onto fresh WFA plates. Isolated fungal strains were maintained on WFA in Petri plates and in 100 ml glass Erlenmeyer flasks at 5 °C. The fungal isolation process was repeated three times on separate occasions using three different samples of aseptically collected weevil frass.
Isolation of Fungus from Weevil Feces
A small amount of aseptically collected pine weevil feces was spread on a WFA Petri plate without adding water and was incubated at room temperature. Fungal strains growing on or beside the fecal pellets were transferred to fresh WFA plates. Fungal isolation was repeated three times using feces collected on three different occasions. Isolated fungal strains were grouped by comparing their morphology and volatile metabolites after culturing them on weevil frass (WF) medium.
Identification of Fungal Strain
Pure fungal strains producing styrene and 3-methylanisole as major volatile organic compounds were streaked on potato dextrose agar (PDA) and yeast extract agar (YEA) media. Using seed cultures from PDA and YEA, fungus was grown for 2 d in liquid medium containing 2 % glucose and 0.5 % yeast extract. The culture was centrifuged to remove the supernatant, and DNA was extracted from the pellet (Müller et al., 1998; Adams and Frostick, 2009). The cells were suspended in phosphate buffer, and an equal weight of glass beads was added. A mixture of phenol, chloroform, and isoamyl alcohol in the ratio 25:24:1 (v/v/v) was added, and the mixture was vortexed for 2 min before centrifugation at 7,500 x g; the supernatant was collected in a fresh tube. The extraction procedure was repeated, and the supernatants were pooled. An equal volume of polyethylene glycol (30 %) was added and the resulting mixture was centrifuged at 7,500 x g. The pellet was suspended in Tris-EDTA (TE) buffer (pH 8.0), reprecipitated with cold ethanol and sodium acetate at −20 °C overnight, centrifuged at 10,000 x g, and the pellet was washed with 70 % ethanol in water before drying and suspending in TE buffer. The concentration of DNA was determined by electrophoresis on 1 % agarose gel with GelRed staining.
The internal transcribed spacer (ITS) of the nuclear DNA was used for the identification of fungus by the polymerase chain reaction (PCR), using universal primers ITS1 (TCC GTA GGT GAA CCT GCG G) and ITS4 (TCC TCC GCT TAT TGA TAT GC) (White et al., 1990; Cardoza et al., 2006; Geib et al., 2008). The reaction conditions were as follows: initial denaturation at 98 °C for 2 min, followed by 98 °C for 10 sec, 55 °C for 30 sec, and 72 °C for 45 sec for 30 cycles, with final extension at 72 °C for 7 min. The reaction product was analyzed by electrophoresis on 1 % agarose gel, and the bands were stained with GelRed and imaged with Gel-Doc (Bio-Rad, USA). The PCR product was sequenced by KTH Genome Center, Biotechnology School, and the sequence was compared by using a Blast search in the NCBI database. Culture identity was confirmed at Centraal Bureau voor Schimmelcultures (CBS), the Netherlands.
Optimization of Volatile Collection by Solid Phase Micro Extraction (SPME)
The collection time for headspace (HS) volatiles was optimized using equal amounts of the major compounds produced by the fungus. Standard mixtures of 0.5 μg/μl styrene and 3-methylanisole were prepared using 1:3 (v/v) dimethyl sulfoxide (DMSO) and water, and 30 μl aliquots of these standard solutions were diluted with water in a 250 ml flask to achieve a final concentration of 15 μg of each compound in 50 ml water. The flask was covered with aluminum foil and equilibrated for at least 15 min prior to collection of the HS volatiles with an SPME fiber (65 μm polydimethylsiloxane/divinylbenzene coating on a stable flex fiber, Supelco, USA), which was conditioned at 250 °C for 30 min before its first use. The SPME needle was introduced into the flask through a pin hole in the aluminum foil, and the fiber was exposed to the HS volatiles for 5, 10, 15, 20, or 30 min at room temperature without shaking; after collection, the fiber was retracted into the needle and injected immediately into the gas chromatograph (GC) at 225 °C. The SPME fiber was cleaned at 225 °C under a stream of helium gas for 5 min before each collection of volatiles. Collection of HS volatiles was repeated in triplicate for each collection time by preparing fresh standard solutions. Based on this study, we selected 15 min as the optimum time for the collection of HS volatiles from inoculated and uninoculated media.
Identification of Volatiles
Volatiles were separated and identified by GC-mass spectrometry (GC-MS) using a Varian 3,400 GC connected to a Finnigan SSQ 7,000 quadrupole MS. The GC was equipped with a split/splitless injector (splitless mode 30 sec), the carrier gas was MS-grade helium with a constant pressure of 10 psi and the column was a SPB-1 capillary (30 m, 0.25 mm ID, and 25 mm film thickness, Supelco USA). The temperature profile was: 40 °C for 30 sec, followed by an increase in temperature of 11 °C/min up to 84 °C for 0.01 min, then an increase of 25 °C/min up to 234 °C, and maintenance at 234 °C for 1.5 min. The injector temperature was set isothermally at 225 °C, and the transfer line connecting the GC to the MS was set to 240 °C throughout the analysis. The filament off time was 0.5 min. The MS ion source temperature was 150 °C; mass spectra were obtained at 70 eV with a mass range of 30 m/z to 600 m/z. Mass spectra of unknown compounds were compared initially to NIST-08 (National Institute of Standard and Technology, USA) MS library. Major compounds were authenticated by analyzing authentic standards on GC-MS using the same parameters as for samples of fungus, and minor compounds were identified by comparing mass spectra and Kovats indexes to published data.
Production of Volatiles from Fungus Growing on Weevil Frass (WF) and Grated Pine Bark (GPB) Broths
To study the profile of the volatiles of isolated fungus, 1 % WF broth was prepared in a 250 ml flask by adding 0.5 g WF to 50 ml of distilled water, and autoclaving for 45 min at 121 °C and 20 psi pressure before inoculation. Three replicates were inoculated with 100 μl spore suspension that was prepared by adding 3 ml sterilized water to fully grown P. expansum on WFA in a Petri dish, followed by mixing and collecting the suspension in a sterilized vial. Three control WF broth replicates were inoculated with 100 μl sterilized water. Similarly, GPB broth was prepared by adding 0.5 g GPB to 50 ml of distilled water in 250 ml flasks, sterilizing, and inoculating as for the WF broth. All flasks were covered with aluminum foil and incubated at room temperature without shaking. Headspace volatiles were collected with SPME for 15 min and analyzed by GC-MS 4, 8, 12, 16, and 20 d after inoculation.
Production of Volatiles from the Fungus Growing on Glucose Yeast Extract (GYE), Grated Pine bark Yeast Extract (GPBYE) and Yeast Extract (YE) Broths
In order to study the effect of additional nutrients on the production of volatiles from the fungus, yeast extract (YE) broth was prepared by adding 5 g YE to 1,000 ml distilled water and distributing 50 ml portions of the broth to 250 ml flasks to which 0.5 g glucose or 0.5 g GPB were added before autoclaving for 15 min at 121 °C and 20 psi. Three flasks from each type of broth culture were then inoculated with 100 μl of fungal spore suspension or sterilized water. Sample and control replicates were incubated at 22 °C (± 2) without shaking. Headspace volatile analysis was carried out using SPME and GC-MS as described above.
Calibration Curve for Styrene and 3-Methylanisole Quantification
To construct standard calibration curves for the quantification of styrene and 3-methylanisole in the HS of fungal inoculated media, a 1:3 solvent mixture of DMSO and water was used to prepare 0.5 μg/μl stock solutions of styrene and 3-methylanisole. Using distilled water and these stock solutions, 50 ml solutions containing 2–20 μg of styrene or 5–45 μg 3-methylanisole were prepared in 250 ml flasks that were covered in aluminum foil and maintained at room temperature for 15 min before collecting and analyzing HS volatiles as described above. The standard curve equation for styrene was y = 2E + 07x with R 2 = 0.9525, and the standard curve equation for 3-methylanisole was y = 1E + 07x with R 2 = 0.9918 based on triplicate determinations for 5 concentrations of each compound.
Bioassay of Pine Weevil Response to Fungal Volatiles
The behavioral responses of both sexes of H. abietis to the fungal volatiles, styrene and 3-methylanisole, were examined in an open multi-choice test arena with 16 odor-baited traps. Volatiles were presented both singly and in combination with conifer host odor (fresh pine twigs). Each of the following four treatments was assigned randomly to four of the 16 traps: (1) pine twig, (2) pine twig + dispenser with tested volatile, (3) dispenser with tested volatile, and (4) empty dispenser. The same treatments were assayed simultaneously in two identical arenas with 50 female weevils in one arena and 50 males in the other. The bioassay was replicated five times for each of the two volatiles and for each sex, a total of 20 separate runs with 50 new weevils each time. Each run lasted 18 h: 9 h light + 6 h dark + 3 h light (fluorescent light from above). The two arenas were kept together in a quiet room with no other activity; the temperature was 20 °C.
The multi choice test arena was made of dark grey Perspex; it was circular in shape with a 1 m diam, and with 10 cm high sides. The sides of the arena were painted with liquid Teflon, Fluon RAD 6 (AG Fluoropolymers, Thornton, Lancashire, UK), which hardens in air to a chemically inert waxy-smooth surface that the insects are unable to climb (Radinovski and Krantz, 1962). In order to eliminate visual cues from the surrounding environment, a 38 cm high wall of brown cardboard, covered with a thin nylon net, surrounded the arena. The 16 traps were uniformly distributed in a circle with a minimum distance of 6.5 cm between traps and 11.5 cm between traps and the side of the arena (Fig. 1a). The traps consisted of slightly conical plastic jars fitted into holes (6 cm diam.) in the floor of the arena, so that the jars extended 3 cm above the arena floor (Fig. 1b). Each jar was capped with a plastic lid under which the dispenser and a piece of pine were suspended out of reach of the weevils. Eight holes (14 mm diam.) with their lower edge 5 mm above the floor of the arena were equally spaced in each jar. Thus, the weevils in the arena had to climb up 5 mm to enter a hole before falling into the trap. The weevils entering a trap fell through a funnel attached to the open bottom of the jar and into a container 40 cm below the arena. Preliminary tests demonstrated that captured weevils did not affect the likelihood of additional weevils entering the traps. The traps were coated with Fluon inside to ensure that all weevils entering the trap slipped down into the container below, where they were collected and counted after each run.
Pine weevils used for the bioassay were collected from sawdust heaps during the migration period in June. Females and males were separated, and weevils were provided with fresh Scots pine bark as food until they were used in the bioassay. Each individual was used only once. At the start of the bioassay, 50 weevils were placed in the center of the arena inside a “release chamber”, a 14 cm diam jar with eight exit holes uniformly spaced along the lower rim. After 15 min the release chamber was carefully lifted out, and the arena was covered with nylon net.
The dispenser used to release the volatiles consisted of a 500 μl Eppendorf tube with a pinhole made with a syringe. Each dispenser was filled with 50 μl of either styrene or 3-methylanisole and was kept at room temperature for 24 h to ensure a constant release rate before use in the orientation bioassay. The release rates were approximately 5 mg of styrene and 1 mg of 3-methylansiole per d. The Scots pine twigs used as the source of natural host odor were collected from a single tree, and the pieces used were of equal size (length 20 mm, 8 mm diam).
Statistical Analyses
Analysis of variance (ANOVA) with Duncan’s post-hoc tests were used to compare the amount of styrene detected in the HS over fungus cultured on various media (WF, GPB, YE, GYE, and GPBYE). Comparisons were made only between treatments analyzed on the same day, using Statistica 10 (Statsoft Inc. USA). Similar comparisons also were made separately for 3-methylanisole production from fungus on various culture media.
The response of female and male pine weevils (total number of weevils captured per treatment and run) to different treatments in the multi-choice bioassay were analyzed separately for each set of 5 experimental runs per tested volatile, using a logistic regression. This was performed by fitting a generalized model (distribution = binomial, link function = logit) to each data set using the procedure PROC GENMOD in SAS® (SAS Institute, version 9.2). In each run, the proportion of weevils captured (= responding) per treatment in relation to the total number of weevils was used as the response variable. To account for over dispersion, the PSCALE option was used in all tests (this fixes the scale parameter to 1 in the estimation process and then assumes the exponential distribution parameter based on the square root of Pearson’s Chi-square statistics/degrees of freedom, df = 16 in all tests). The statistical differences between treatment levels were estimated based on differences in Least Square Means and the corresponding Chi-square statistics. The treatment pine twig was used as treatment reference category.
Results
Isolation and Identification of the Fungal Strain
To isolate the fungal strain present in the frass of H. abietis, moist WF was incubated at room temperature to mimic the natural conditions at oviposition sites. Under these conditions, the fungi growing on the frass surface became visible after 3 d. Fungus that produced styrene and 3-methylanisole was isolated from both frass and feces. By examining its morphology and sequencing DNA in the ITS1 and ITS4 regions, the fungus was identified as Penicillium expansum Link ex. Thom. Identification was confirmed by the CBS-KNAW Fungal Biodiversity Center using ITS1 & ITS2 and the BT (partial fragment of the beta-tubulin gene) region. The strain showed 100 % sequence identity with P. expansum based on comparisons to Genbank and CBS databases.
Optimum Collection Time for HS volatiles
The optimum collection time for the SPME fiber was evaluated using standard solutions of styrene and 3-methylanisle. The amount of styrene and 3-methylanisole adsorbed onto the SPME fiber increased linearly during 15 min of exposure to HS volatiles. After this time, the amount of styrene adsorbed onto the SPME fiber decreased gradually whereas 3-methylanisole continued to be adsorbed in a linear fashion (Supplementary Fig. 1).
Fungal Volatiles Analysis
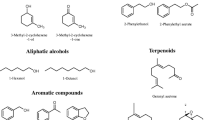
Headspace analysis of the flask containing the WF broth that had not been inoculated (control broth) demonstrated the presence of α-pinene, β-pinene, 3-carene, o-cymene, m-cymene limonene, heptanal, 1-octene-3-one, octanal, octenal, and nonanal (Fig. 2). The HS of P. expansum cultured on WF broth contained large amounts of styrene and 3-methylanisole and a very small amount of anisole. Although the HS of the inoculated broth contained the monoterpenes found in the control, none of the aldehydes or ketone found in the HS of the control were found in the HS of the WF medium inoculated with fungus (Fig. 2). The HS analyses of fungus grown on GPB and GPBYE media were similar to the HS for fungus grown on WF (Fig. 2) with the exception of anisole, which was observed only in the HS of fungus grown on the WF medium.
Styrene and 3-Methylanisole Production from P. expansum Cultures
The P. expansum strain isolated from weevil frass produced styrene and 3-methyanisole as major volatile compounds when cultured on various media containing different nutrients and toxic compounds (Figs. 3 and 4). Production of styrene and 3-methylanisole was compared over 20 days by culturing the fungus on wood related waste material and glucose e.g., WF, GPB, GPBYE, GYE, and YE liquid culture media. The amount of styrene and 3-methylanisole produced by the fungus differed according to the substrate used (Figs. 3 and 4). The amount of styrene produced was highest for fungus cultured on grated pine bark in combination with 0.5 % nutrient rich yeast extract (GPBYE). The amount of styrene produced by the fungus grown on GPBYE was slightly (1.5 times) higher than from YE alone, 5 times higher than from GYE, 10 times higher than from GPB, and 2.5 times higher than from fungus grown on the WF medium (Fig. 3). Similarly, the amount of 3-methylanisole produced by the fungus cultured on GPBYE was also highest compared to fungus cultured on all other media used in this study (Fig. 4). In total, fungus grown on GPBYE produced 56 times higher amounts of 3-methylanisole than from fungus on GPB, 2.3 times higher than from WF, 1.9 times higher than from GYE and 1.4 times higher than from fungus on YE medium (Fig. 4).
Amount of styrene detected in the headspace over Penicillium expansum grown on five different [weevil frass (WF), grated pine bark (GPB), yeast extract (YE), glucose yeast extract (GYE), and grated pine bark yeast extract (GPBYE)] culture media. Vertical bars indicate standard error for N=3; columns with different letters are significantly different (P<0.05). Comparison of styrene production was carried out between different culture media analyzed on the same day
Amount of 3-methylanisole detected in the headspace over Penicillium expansum grown on five different [weevil frass (WF), grated pine bark (GPB), yeast extract (YE), glucose yeast extract (GYE), and grated pine bark yeast extract (GPBYE)] culture media. Vertical bars indicate standard error for N=3; columns with different letters are significantly different (P < 0.05). Comparison of 3-methylanisole production was carried out between different culture media analyzed on the same day
Response of the Pine Weevils to Styrene and 3-Methylanisole
Styrene clearly reduced the attractiveness of pine twig odor to both female and male pine weevils (Fig. 5). About twice as many weevils entered traps with only pine twigs compared to traps containing both pine and styrene; this difference was significant for both sexes (χ2 = 12.88, df = 1, P = 0.001 for females, χ2 = 15.89, df = 1, P < 0.001 for males). There was no difference in response between styrene alone and the control (Fig. 5).
Mean number of pine weevils (Hylobius abietis) captured in traps with different baits in a multi choice bioassay, examining the effect of styrene. Columns with different letters are significantly different to each other when comparison was employed between treatments for male and female weevils independently. Error bars = SE
In contrast to styrene, 3-methylanisole only reduced attraction to pine odor in males, but this reduction was as high as 63 % (Fig. 6). Thus, in males, the pine twig treatment differed significantly from pine plus 3-methylanisole (χ2 = 17.17, df = 1, P < 0.001) and also from the other two treatments. For females, the pine treatment only differed significantly from 3-methylanisole alone (χ2 = 4.09, df = 1, P < 0.043) and the empty control (χ2 = 5.98, df = 1, P < 0.015). There was no effect of pure 3-methylanisole odor on weevil attraction compared to the control (Fig. 6).
Mean number of pine weevils (Hylobius abietis) captured in traps with different baits in a multi choice bioassay, examining the effect of 3-methylanisole. Columns with different letters are significantly different to each other when comparison was employed between treatments for male and female weevils independently. Error bars = SE
Discussion
Plants and microbes produce a variety of chemicals that potentially could serve as ecologically sustainable alternatives to synthetic insecticides. In this study we examined the volatile metabolites of a fungus present in H. abietis feces and frass in order to identify compounds that repel the insects. A strain of P. expansum was isolated from the frass/feces that H. abietis deposit at the oviposition site, presumably thus protecting their eggs from feeding conspecifics (Nordlander et al., 1997). The fungus produced large amounts of styrene and 3-methylanisole when cultured on its natural medium, weevil frass. Styrene was detected in the weevil frass medium 4 d after fungal inoculation, and the production lasted for more than 20 d at 20 °C. This should be enough time to protect pine weevil eggs until larval hatching, which takes 1–4 wk at temperatures between 20 and 10 °C (Eidmann, 1974). Weevil frass culture medium inoculated with fungus provides conditions similar to those occurring naturally around the oviposition site.
SPME is a technique for collecting a wide range of volatile and semi-volatile compounds without the laborious work of solvent extraction and concentration, which may result in the loss of very volatile compounds (Zhang and Pawliszyn, 1993). The SPME fiber has a number of binding sites that have different affinities for different compounds. The optimum time required to collect HS volatiles by SPME depends on many factors, including the nature of the compound to be collected (Agelopoulos and Pickett, 1998), the type of fiber, the adsorption time, and the temperature (Barua et al., 2008). Under the conditions used here, at concentrations of compounds similar to those present in the natural samples, the optimum adsorption time for the major volatile metabolites of P. expansum, namely styrene and 3-methylanisole, was 15 min. In 2008, Barua et al. presented similar results for styrene in serum and blood samples.
Penicillium expansum usually is found on decomposing material in soil, but also has been reported in rodent feces (Stejskal et al., 2005). Styrene and 3-methylanisole are produced by P. expansum and a number of other fungal species that were isolated from a compost facility, and cultured on yeast extract sucrose agar media (Fischer et al., 1999). Styrene and 3-methylanisole in the HS of samples of spoiled apples were associated with the toxic compound patulin produced by P. expansum (Karlshoj et al., 2007). Similarly, Sagi-Kiss and Fodor (2011) reported the presence of styrene and 3-methylanisole as a P. expansum infection marker for plums, and studied their production over a period of 4 days using plum agar and malt extract agar media.
The production of styrene and 3-methylanisole is dependent on the availability of suitable food and culturing conditions. In a study involving the cultivation of two strains of P. expansum, styrene and 3-methylanisole were found only when malt extract agar and yeast extract glucose chloramphenicol agar were used as the culture media; the fungal strains did not produce these compounds when grown on a broth made from wood material (Fiedler et al., 2001). In a previous study, P. expansum was reported to produce isobutanol, isopentanol, α-bergamotene and a small amount of 3-methylanisole when grown on yeast extract sucrose agar medium (Larsen and Frisvad, 1995). Penicillium sp. isolated from cinnamon samples converted cinnamon related compounds to styrene (Lafeuille et al., 2009). Styrene is also produced by other fungal species such as Penicillium caseifulvum (Larsen, 1998) and Trichoderma on potato dextrose broth with aromatic cinnamic acid added (Pinches and Apps, 2007).
The P. expansum strain used in this study did grow on wood bark, and produced styrene and 3-methylanisole. When we added YE as a supplement, the strain produced rather large amounts of styrene and 3-methylansisole compared to all other tested media. Both aromatic amino acids and various sugars can be used by microbes as a source for biosynthesis of the volatiles (Pagot et al., 2007; Pinches and Apps, 2007). The readily available free aromatic amino acids might be converted first to volatiles, which would explain why the fungus grown on GPBYE medium produced a 10–50 times more of the volatiles compared to the fungus on GPB, in which most of the sugars and aromatic precursors are bound to lignin. Furthermore, GPB medium contains a large amount of terpenoids and phenolics that may inhibit the fungal growth. In the case of GPBYE, readily available nutrients from YE could help the fungus to grow and overcome the potential toxicity of terpenoids and phenolics from the bark. In a previous study, conditions for the production of styrene by Fusarium oxysporum grown on potato dextrose were optimized, and it was suggested that glucose was the precursor and central to the biosynthetic scheme for styrene production (Beck et al., 2008).
The volatiles (+)-α-pinene, (−)-α-pinene, and (+)-3-carene (Wibe et al., 1997) are emitted by pine, and are highly attractive to the pine weevil (Nordlander, 1990). Styrene masked the attractive odor of freshly cut pine twigs for both sexes of pine weevil. 3-Methylsanisole had a similar effect, but only for male pine weevils; the reason for this difference in response between the sexes is unknown. Many insects are deterred from interacting with fungal infected food (Rayamajhi et al., 2006). Most commonly, insects detect fungal infection in foods by chemical signals as well as via visual cues (Röder et al., 2007). The alpine leaf beetles, Oreina elongata and O. cacaliae avoid feeding and ovipositing on host plants with clear patches of fungal infection as well as on infected plants without any clear sign of infection. Chemical signals involved in this communication have not yet been studied (Röder et al., 2007). Fungi produce toxins and insects might identify toxin containing or spoiled food by detecting volatiles produced by fungi.
Waste material from the forestry industry mainly consists of material similar to grated wood bark and could be a valuable source for producing both pine weevil repellent and the monomer styrene for sustainable styrene polymer production. This fermentation process could be conducted before forest waste materials are used as biofuel. From the present investigation, it is clear that there is potential for the production of important insect controlling compounds using “green chemistry” alternatives.
References
Adams, J. D. W. and Frostick, L. E. 2009. Analysis of bacterial activity, biomass and diversity during windrow composting. Waste Manage. 29:598–605.
Agelopoulos, N. G. and Pickett, J. A. 1998. Headspace analysis in chemical ecology: Effects of different sampling methods on ratios of volatile compounds present in headspace samples. J. Chem. Ecol. 24:1161–1172.
Barua, R., Chi, L.-H., Fitzpatrick, R., Gillard, D., and Kostyniak, P. J. 2008. Determination of volatile organic compounds in biological samples using headspace solid-phase microextraction and gas chromatography: Toluene and styrene. J. Anal. Toxicol. 32:379–386.
Beck, J. J., Merrill, G. B., Palumbo, J. D., and O’Keeffe, T. L. 2008. Strain of Fusarium oxysporum isolated from almond hulls produces styrene and 7-methyl-1,3,5-cyclooctatriene as the principal volatile components. J. Agric. Food Chem. 56:11392–11398.
Bohman, B., Nordlander, G., Nordenhem, H., Sunnerheim, K., Borg-Karlson, A. K., and Unelius, C. R. 2008. Structure-activity relationships of phenylpropanoids as antifeedants for the pine weevil Hylobius abietis. J. Chem. Ecol. 34:339–352.
Borg-Karlson, A. K., Nordlander, G., Mudalige, A., Nordenhem, H., and Unelius, C. R. 2006. Antifeedants in the feces of the pine weevil Hylobius abietis: Identification and biological activity. J. Chem. Ecol. 32:943–957.
Cardoza, Y. J., Klepzig, K. D., and Raffa, K. F. 2006. Bacteria in oral secretions of an endophytic insect inhibit antagonistic fungi. J. Ecol. Entomol. 31:636–645.
Daisy, B. H., Strobel, G. A., Castillo, U., Ezra, D., Sears, J., Weaver, D. K., and Runyon, J. B. 2002. Naphthalene, an insect repellent, is produced by Muscodor vitigenus, a novel endophytic fungus. Microbiology 148:3737–3741.
Day, K. R., Nordlander, G., Kenis, M., and Halldorson, G. 2004. General biology and life cycles of bark weevils, pp. 331–349, in F. Lieutier, K. R. Day, A. Battisti, J.-C. Gregoire, and H. F. Evans (eds.), Bark and wood boring insects in living trees in Europe, a synthesis. Kluwer Academic Publishers, Dordrecht.
Dong, J., Zhu, Y., Song, H., Li, R., He, H., Liu, H., Huang, R., Zhou, Y., Wang, L., Cao, Y., and Zhang, K. 2007. Nematicidal resorcylides from the aquatic fungus Caryospora callicarpa YMF1.01026. J. Chem. Ecol. 33:1115–1126.
Eidmann, H. H. 1974. Hylobius Schönh, pp. 272–293, in W. Schwenke (ed.), Die Forstschadlingc Europas, Band 2. Verlag Paul Parey Hamburg, Berlin.
Fiedler, K., Schutz, E., and Geh, S. 2001. Detection of microbial volatile organic compounds (MVOCs) produced by moulds on various materials. Int. J. Hyg. Environ. Health 204:111–121.
Fischer, G., Schwalbe, R., Möller, M., Ostrowski, R., and Dott, W. 1999. Species-specific production of microbial volatile organic compounds (MVOC) by airborne fungi from a compost facility. Chemosphere 39:795–810.
Geib, S. M., Filley, T. R., Hatcher, P. G., Hoover, K., Carlson, J. E., Jimenez-Gasco, M. D., Nakagawa-Izumi, A., Sleighter, R. L., and Tien, M. 2008. Lignin degradation in wood-feeding insects. Proc. Natl. Acad. Sci. U. S. A. 105:12932–12937.
Hotson, I. K. 1982. The avermectins—a new family of antiparasitic agents. J. S. Afr. Vet. Assoc. 53:87–90.
Karlshoj, K., Nielsen, P. V., and Larsen, T. O. 2007. Prediction of Penicillium expansum spoilage and patulin concentration in apples used for apple juice production by electronic nose analysis. J. Agric. Food Chem. 55:4289–4298.
Kolmodin-Hedman, B., Akerblom, M., Flato, S., and Alex, G. 1995. Symptoms in forestry workers handling conifer plants treated with permethrin. Bull. Environ. Contam. Toxicol. 55:487–493.
Lafeuille, J. L., Buniak, M. L., Vioujas, M. C., and Lefevre, S. 2009. Natural formation of styrene by cinnamon mold flora. J. Food Sci. 74:M276–M283.
Lam, K., Tsang, M., Labrie, A., Gries, R., and Gries, G. 2010. Semiochemical-mediated oviposition avoidance by female house flies, Musca domestica on animal feces colonized with harmful fungi. J. Chem. Ecol. 36:141–147.
Larsen, T. O. 1998. Volatile flavor production by Penicillium caseifulvum. Int. Dairy J. 8:883–887.
Larsen, T. O. and Frisvad, J. C. 1995. Characterization of volatile metabolites from 47 Penicillium-taxa. Mycol. Res. 99:1153–1166.
Legrand, S., Nordlander, G., Nordenhem, H., Borg-Karlson, A.-K., and Unelius, C. R. 2004. Hydroxy-methoxybenzoic methyl ester: Synthesis and antifeedant activity on the pine weevil, Hylobius abietis. Z. Naturforsch., B: Chem. Sci. 59:829–835.
Müller, F. M. C., Werner, K. E., Kasai, M., Francesconi, A., Chanock, S. J., and Walsh, T. J. 1998. Rapid extraction of genomic DNA from medically important yeasts and filamentous fungi by high-speed cell disruption. J. Clin. Microbiol. 36:1625–1629.
Nordlander , G. 1990. Limonene inhibits attraction to α-pinene in the pine weevils Hylobius abietis and H. pinastri. J. Chem. Ecol. 16:1307-20.
Nordlander, G., Nordenhem, H., and Bylund, H. 1997. Oviposition patterns of the pine weevil Hylobius abietis. Entomol. Exp. Appl. 85:1–9.
Pagot, Y., Belin, J.-M., Husson, F., and Spinnler, H.-E. 2007. Metabolism of phenylalanine and biosynthesis of styrene in Penicillium camemberti. J. Dairy Res. 74:180–185.
Peshin, R., Dhawan, A., and Pimentel, D. 2009. Environmental and economic costs of the application of pesticides primarily in the United States. pp. 89–111 in Integrated Pest Management: Innovation-Development Process. Springer Netherlands.
Peterson, R. A., Bradner, J. R., Roberts, T. H., and Nevalainen, K. M. H. 2009. Fungi from koala (Phascolarctos cinereus) faeces exhibit a broad range of enzyme activities against recalcitrant substrates. Lett. Appl. Microb. 48:218–225.
Petersson, M. and Örlander, G. 2003. Effectiveness of combinations of shelterwood, scarification, and feeding barriers to reduce pine weevil damage. Can. J. For. Res. 33:64–73.
Pinches, S. E. and Apps, P. 2007. Production in food of 1,3-pentadiene and styrene by Trichoderma species. Int. J. Food Microbiol. 116:182–185.
Radinovski, S. and Krantz, G. W. 1962. The use of Fluon to prevent the escape of stored-product insects from glass containers. J. Econ. Entomol 55:815–816.
Rayamajhi, M. B., Van, T. K., Pratt, P. D., and Center, T. D. 2006. Interactive association between Puccinia psidii and Oxyops vitiosa, two introduced natural enemies of Melaleuca quinquenervia in Florida. Biol. Control. 37:56–67.
Röder, G., Rahier, M., and Naisbit, R. E. 2007. Coping with an antagonist: the impact of a phytopathogenic fungus on the development and behaviour of two species of alpine leaf beetle. Oikos 116:1514–1523.
Sagi-Kiss, V. and Fodor, P. 2011. Development of a SPME-GC-MS method for spoilage detection in case of plums inoculated with Penicillium expansum. Acta Aliment. 40:188–197.
Stejskal, V., Hubert, J., Kubátová, A., and Váñová, M. 2005. Fungi associated with rodent feces in stored grain environment in the Czech Republic. J. Plant Dis. Protect. 112:98–102.
Sun, J., Wang, H., Lu, F., Du, L., and Wang, G. 2008. The efficacy of nematicidal strain Syncephalastrum racemosum. Ann. Microbiol. 58:369–373.
Sunnerheim, K., Nordqvist, A., Nordlander, G., Borg-Karlson, A.-K., Unelius, C. R., Bohman, B., Nordenhem, H., Hellqvist, C., and Karlen, A. 2007. Quantitative structure-activity relationships of pine weevil antifeedants, a multivariate approach. J. Agric. Food Chem. 55:9365–9372.
Unelius, C. R., Nordlander, G., Nordenhem, H., Hellqvist, C., Legrand, S., and Borg-Karlson, A.-K. 2006. Structure-activity relationships of benzoic acid derivatives as antifeedants for the pine weevil, Hylobius abietis. J. Chem. Ecol. 32:2191–2203.
White, T. J., Bruns, T., Lee, S., and Taylor, J. W. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, pp. 315–322, in M. A. Innis, D. H. Gelfand, J. Sninsky, and T. J. White (eds.), PCR protocols: A guide to methods and applications. Academic, New York.
Wibe, A., Borg-Karlson, A. K., Norin, T., and Mustaparta, H. 1997. Identification of plant volatiles activating single receptor neurons in the pine weevil (Hylobius abietis). J. Comp. Physiol. A 180:585–595.
Zhang, Z. Y. and Pawliszyn, J. 1993. Headspace solid-phase microextraction. Anal. Chem. 65:1843–1852.
Acknowledgments
We gratefully acknowledge financial support from Formas to AKBK, GN and GKR, from the Mobilitas Programme Top Researcher Grant No. MTT2 “Chemical Ecology” to AKBK, and from the Higher Education Commission, Government of Pakistan for the Ph.D. scholarship to MA. MA is thankful to Kazuhiro Nagahama for introducing him to practical microbiology. We are very grateful to Helena Bylund and Raimondas Mozuraitis for advice on statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplemental Fig. 1
Effect of HS-SPME collection time on the amounts of styrene and 3-methylanisole adsorbed on the SPME fiber from the HS of a standard solution in 50 ml water in an E-flask. Data points are means of three independent determinations, and error bars indicate standard deviation. (DOCX 34.8 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Azeem, M., Rajarao, G.K., Nordenhem, H. et al. Penicillium expansum Volatiles Reduce Pine Weevil Attraction to Host Plants. J Chem Ecol 39, 120–128 (2013). https://doi.org/10.1007/s10886-012-0232-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-012-0232-5