Abstract
We report the identification, synthesis, and field bioassays of a female-produced sex attractant pheromone component of the cerambycid beetle Ortholeptura valida (LeConte). Headspace volatiles from females contained a female-specific compound, (Z)-11-octadecen-1-yl acetate, which elicited a strong response from antennae of adult males in coupled gas chromatography-electroantennogram analyses. In field bioassays, significant numbers of males were collected by traps baited with this compound. The pheromone represents a new structural class of cerambycid pheromones, and is the first pheromone identified for a cerambycid species in the subfamily Lepturinae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerambycid beetles of the subfamily Lepturinae (sensu Napp, 1994) are primarily Holarctic in distribution (Linsley, 1959; Švácha and Danilevsky, 1986), and the larvae typically develop in decaying wood (Linsley, 1959; Linsley and Chemsak, 1972; Švácha and Danilevsky, 1988). The adults of many species are diurnal and feed on the pollen or nectar of flowers (Linsley, 1961; Linsley and Chemsak, 1972). Other than those general habits, however, little is known of the behavior of most lepturine species (e.g., Swaine and Hopping, 1928; Michelsen, 1963; Linsley and Chemsak, 1972; Švácha and Danilevsky, 1988). We previously have suggested that mate location in lepturines is mediated by host plant volatiles (e.g., see Hanks, 1999; Ray, 2009). To our knowledge, long-range pheromones have not been reported for any species in the subfamily. Adult males of some lepturine species mate with females only after contacting them with their antennae (Michelsen, 1963), suggesting that mate recognition is mediated by contact pheromones (Millar et al., 2009).
We report here the identification, synthesis, and field bioassays of a female-produced sex attractant pheromone component of the lepturine Ortholeptura valida (LeConte). The active compound, (Z)-11-octadecen-1-yl acetate, represents the first example of a new structural class of pheromones within the Cerambycidae. Ortholeptura valida is endemic to the Pacific coast of North America, and occurs from British Columbia to southern California along the Cascade, Sierra Nevada, Transverse, and Peninsular mountain ranges (Linsley and Chemsak, 1976; Švácha and Danilevsky, 1986, 1988). The larvae develop in the decaying trunks of conifers of many species (Craighead, 1923; Linsley and Chemsak, 1976; Cope, 1984; MacRae and Rice, 2007). Adults emerge from late June to August, and mate and oviposit on trees that are stressed, moribund, or have recently died (RLA, pers. obs.; Linsley and Chemsak, 1976). Ortholeptura valida is unusual among lepturines in that the adults are nocturnal (Linsley and Chemsak, 1976), and apparently do not feed on flowers (e.g., Švácha and Danilevsky, 1988). Adults often are collected at lights at night (Linsley and Chemsak, 1976).
Methods and Materials
Source of Beetles
We collected prepupal larvae of O. valida on 3 June 2010 from downed Abies concolor Lind. & Gord. in the Barton Flats region of the San Bernardino National Forest (along California State Route 38, Mountaintop Ranger District, San Bernardino Co., CA, USA; N 34° 10′ 6″ W 116° 53′ 31″, 1940 m elevation). Dominant vegetation included mature A. concolor, Pinus jeffreyi Grev. & Balf., P. ponderosa Dougl. ex P. & C. Lawson, and Quercus kelloggii Newb. We extracted larvae from decomposing wood with an axe, and placed them in 25-ml plastic vials. In the laboratory, artificial pupation chambers were constructed from newsprint that was rolled into tubes (~7 cm long, ~2 cm diam). We placed a single larva in each tube, plugged the ends with tissue paper, misted them with water, and stored tubes together in a 4 l plastic bag at room temperature. Tubes were examined 2–3 times per week to monitor development.
The beetles eclosed during 20 June–5 July 2010. Teneral adults were returned to their paper tubes and temporarily stored at 4°C. Beetles were sexed using the sexually-dimorphic characters of antennal length and shape of the terminal abdominal sternite (Linsley and Chemsak, 1976). Two beetles of each sex were prepared for aeration by transferring them into individual vials (20 ml), and leaving them under ambient room conditions for 24 hr. The adults were virgins, and their cuticles were not fully sclerotized at the time that they were aerated.
Collection of Volatiles
We collected headspace odors of adult male and female O. valida in an environmentally controlled room (26°C, 65% RH). Individual beetles were placed in modified 250 ml Ball® Mason-style canning jars that contained a glass vial (~2 ml) of 10% sucrose solution plugged with cotton, as nourishment. The jar lids were fitted with a Teflon® liner and two brass bulkhead unions (pipe thread, ~0.64 cm to 0.32 cm; Swagelok®, San Diego Valve and Fitting Co., San Diego CA, USA), one of which was connected to a flow meter-controlled vacuum source, and the other to a charcoal scrubber. Collectors were 6-mm OD by 41 mm long glass tubes containing a 5 mm long bed of thermally-desorbed activated charcoal (200 mesh; Fisher Scientific, Pittsburgh, PA, USA) held in place by Soxhlet-extracted (ether) glass wool plugs. The collectors were attached to jar lids with brass fittings (0.64 cm) with Teflon® ferrules (Swagelok®). Charcoal-filtered air was pulled through the chamber and collector at ~160 ml/min. Aerations were run for 4 days, after which the collectors were extracted with dichloromethane (3 rinses, for a total volume of 500 μl). Extracts were stored in a freezer (−4°C).
Coupled Gas Chromatography-Electroantennogram Detection (GC-EAD) and Gas Chromatography–Mass Spectrometry (GC-MS) Analyses
GC-EAD analyses were performed using DB-5 and DB-Wax columns (both 30 m × 0.25 mm i.d., 0.25 μm film; J&W Scientific, Folsom, CA, USA). The carrier and makeup gas was helium. The GC oven was programmed from 40°C for 1 min, 10°C (5°C for calculation of Kovat’s indices [KI] on DB-Wax) per min to 275°C for DB-5 and 250°C for DB-Wax, hold for 45 min. Solvent extracts (1 μl aliquots) were analyzed in splitless mode. The effluent from the columns was split with half of the sample going to the FID detector and the other half to the EAD. The portion directed to the EAD was diluted in a humidified air stream that was directed over the antennal preparation that consisted of the excised terminal 4–5 antennal segments of a male beetle, with the distal tip cut off with a razor blade, placed between two saline-filled glass capillary electrodes (7.5 g NaCl, 0.21 g CaCl2, 0.35 g KCl, and 0.20 g NaHCO3 in 1 l Milli-Q® purified water). The glass electrodes were fitted with 0.2 mm diam gold wires that connected to the amplifier, with the amplifier gain set at 100X amplification. A single antennal preparation was used for as many as ten runs. GC-MS analyses were carried out with an Agilent 6890 N GC interfaced to a 5975 C mass selective detector (Agilent, Santa Clara, CA, USA). The GC was fitted with an HP5-MS column (30 m × 0.25 mm i.d., 0.25 μm film), and the same temperature program and injection conditions were used as described above. KI were calculated for unknowns and standards relative to blends of straight-chain hydrocarbons. For increased precision, KI values on the DB-Wax column were obtained using a GC oven program rate of 5 rather than 10°C/min.
Epoxidation of the Unknown
Approximately half of an aeration extract from a female beetle was treated with 20 μl of a solution of m-chloroperbenzoic acid in methylene chloride (2 mg/ml), and the solution was held at room temperature for 1 hr, swirling occasionally. The mixture was then concentrated just to dryness under a stream of nitrogen, the residue was taken up in pentane, and loaded onto a small column of silica gel (2 cm × 3 mm ID). The column was eluted successively with pentane (400 μl) and 2 × 300 μl 7.5% EtOAc in pentane. The resulting fractions were concentrated to ~25 μl and analyzed by GC-MS as described above. The epoxidized pheromone was in the third fraction.
Synthesis of (Z)-11-Octadecen-1-yl acetate
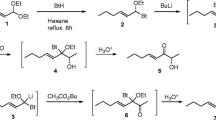
Lithium aluminum hydride (LiAlH4, 150 mg, 4 mmol) was added to 10 ml of dry THF under argon, and the mixture was cooled in an ice bath while a solution of cis-vaccenic acid (300 mg, 1.06 mmol; Sigma Chemical Co., St. Louis, MO, USA) in 3 ml THF was added dropwise. When the addition was complete, the ice bath was removed and the mixture was stirred overnight. The mixture was cooled in an ice bath and quenched by cautious addition of water (0.16 ml; foams!), 2 M NaOH (0.12 ml), and water (0.56 ml). The resulting slurry was stirred 15 min, during which time the inorganic salts precipitated out as a gummy white solid. The mixture was filtered through a short pad of Celite® (Fisher Scientific, Fairlawn, NJ, USA), rinsing the solids well with ether. The filtrate was dried over anhydrous Na2SO4, and then concentrated on a rotary evaporator. The resulting alcohol was taken up in a solution of 25 ml dry ether, and pyridine (160 μl, 2 mmol) and acetyl chloride (145 μl, 2 mmol) were added. The solution was stirred overnight at ~22°C, then poured into water and extracted with ether. The ether extract was washed with brine, dried over anhydrous Na2SO4, concentrated, and Kugelrohr distilled (oven temp. to 110°C, 0.05 mm), yielding (Z)-11-octadecen-1-yl acetate (270 mg, >99% pure by GC). The proton NMR spectrum was in agreement with that previously reported (Vig et al., 1988).
Field Bioassays of Synthetic Compounds
We tested the response of adult O. valida to the female-specific component of the aeration, (Z)-11-octadecen-1-yl acetate, in field bioassays. We used black flight-intercept panel traps (1.2 high × 0.3 m wide, model PT Intercept™, APTIV, Portland, OR, USA). Internal surfaces of traps were treated with Fluon®, which renders traps more slippery and can greatly improve trap catch of cerambycid beetles (Graham et al., 2010). To capture beetles alive, traps were modified by replacing the collection basin with a 1.9 l plastic funnel that was attached to a clear plastic jar (1.9 l, General Bottle Supply Company, Los Angeles, CA, USA). The funnel spout was cut off, leaving a 3.5-cm-diam hole at the bottom, a ~10 cm hole was cut into the threaded lid of the jar, and the funnel spout was hot-melt glued to the lid so that the spout was inside the jar. We hung traps from tree branches or from L-shaped hangers constructed of PVC pipe (as in Graham et al., 2010) with the trap bases ~30 cm above the ground. Pheromone lures were clear low-density polyethylene press-seal bags (“Zipper” seal sample bags, 2” × 3”, 51 μm wall thickness, #01-816-1A; Fisher Scientific, Pittsburgh, PA, USA) loaded with 25 mg of (Z)-11-octadecen-1-yl acetate in 475 μl of absolute ethanol (500 μl total volume) and sealed. Control lures contained 500 μl of absolute ethanol. Lures were suspended with wire in the central open area of traps.
We monitored flight activity of O. valida with two pheromone-baited sentinel traps at the Barton Flats study site. The sentinel traps were set up on 28 July 2010, within the seasonal activity period of the species (Linsley and Chemsak, 1976). Seven adult male O. valida were captured by sentinel traps within 2 days, and the field bioassay then was set up and run until 25 August 2010 (clear skies, no precipitation, air temperatures 10–33°C, maximum wind speed 11 km/h). We set up three replicates of the two treatments at the Barton Flats site, and two replicates at another site ~4 km to the southwest (along West Jenks Lake Road; N 34° 9′ 47″ W 116° 53′ 57″, 2020 m elevation). At both sites, traps were positioned in a southerly transect and separated by ~10 m, with pheromone and control treatments assigned randomly to traps.
Traps were checked for beetles at intervals of 4–6 days, and were shifted along transects every 7 days to control for location effects, at which time lures were replaced. Captured beetles were sexed using the sexually-dimorphic characters listed above. Differences between trap treatments in the number of beetles captured per trap per time interval were tested with the nonparametric Friedman’s test (blocked by date and site; PROC FREQ with CMH option; SAS Institute, 1999) because assumptions of analysis of variance were violated by heteroscedasticity (Sokal and Rohlf, 1995). We excluded from the analysis one date on which no beetles were captured.
Voucher specimens of O. valida have been deposited at the Entomology Research Museum at the University of California, Riverside (museum voucher numbers: UCRC ENT 00282753, 00282754, 00282755, 00282756, 00282757, 00282758).
Results
Identification of the Pheromone
Analyses of the extracts of volatiles released by female O. valida showed that a single sex-specific trace component in the extracts elicited a strong and reproducible response from antennae of male beetles (Fig. 1), with a KI on the relatively nonpolar DB-5 column of 2192 and a KI on the polar DB-Wax column of 2512. The EI mass spectrum showed a series of regularly spaced ion clusters separated by ~14 mass units, suggestive of a long hydrocarbon chain (Fig. 2, top). The values of the most prominent ions in each cluster (m/z 69, 82, 96, 109, 123, etc.) suggested that the compound was unsaturated, or that it had a functional group that was readily lost as a neutral fragment to leave an unsaturated ion that fragmented further. A prominent fragment at m/z 61 suggested the presence of an acetate. The highest mass ion observed was at m/z 250 (possible formula, C18H34), which could reasonably be derived from the loss of acetic acid from a monounsaturated 18 carbon acetate ester.
Coupled gas chromatography-electroantennogram analysis of an extract of headspace odors from a female Ortholeptura valida. Upper trace is the chromatogram; lower inverted trace is the electroantennogram signal from the antenna of a male beetle. DB-5 column, 40°C/1 min, then 10°C/min to 275°C, hold 40 min. Scale bar represents 5 mV for the amplified antennal response (gain 100X)
EI mass spectra (70 eV) of the compound that elicited a strong electroantennogram response in Fig. 1 (top) and of an authentic standard of (Z)-11-octadecen-1-yl acetate
GC-MS analyses of the model compound (Z)-9-octadecen-1-ol (oleyl alcohol) revealed that its mass spectrum was quite similar to that of the unknown, but the Kovats index (2062) on the DB-5 column was 130 units less than that of the unknown. However, a standard of (Z)-9-octadecen-1-yl acetate gave a retention index (2186) and a mass spectrum that was similar, but not an exact match to that of the unknown, indicating that the unknown was probably one of the other octadecenyl acetate isomers. The double bond position and geometry were determined by epoxidation of an extract, followed by GC-MS analysis. The resulting derivative gave enhanced fragments at m/z 127 (C8H15O+) and 241 (C14H25O +3 ) from cleavage on either side of the epoxide, identifying the double bond in the parent compound as being in the 11 position (Fig. 3, top). An exact retention time match of the derivative with an epoxidized standard of (Z)-11-octadecen-1-yl acetate (vaccenyl acetate, Z11-18:Ac) indicated that the double bond in the unknown had the Z-configuration (cis- and trans-epoxides from cis- and trans-alkenes, respectively, have different retention times; Attygalle, 1998). This was confirmed by the presence of a trace amount of the corresponding trans-epoxide in the synthetic standard; the cis- and trans-epoxides were separated to baseline on the DB-5 column (cis: retention time 22.70 min; trans: 22.56 min). The epoxidized insect extract showed a peak only at 22.70 min, indicating that the insect produced only the Z-stereoisomer. The identification was confirmed by exact matches in retention times between the unknown and Z11-18:Ac on both the DB-5 and DB-WAX columns, and matching mass spectra (Fig. 3). Thus, the highest mass ion at m/z 250 observed in EI mass spectrum of the parent compound did indeed correspond to loss of acetic acid from the molecular ion (m/z 310), as commonly occurs with long-chain acetates under 70 eV electron impact ionization conditions.
EI mass spectra (70 eV) of the epoxide of the compound that elicited a strong EAD response in Fig. 1 (top) and of epoxidized authentic (Z)-11-octadecen-1-yl acetate
Field Bioassays of Synthetic Compounds
Traps baited with pheromone lures captured 139 and 41 male O. valida (a total of 180 beetles) at the Barton Flats and Jenks Road sites, respectively, but no beetles were captured by control traps. As many as 46 male beetles were captured per pheromone trap per date, with a mean of 7.5 ± 1.9 beetles (SE; significantly different from the 0 mean for controls; Friedman’s Q 1,48 = 28.7, P < 0.001). Traps baited with the synthetic pheromone did not capture any other insect species.
Discussion
Exclusive attraction of O. valida males to lures baited with synthetic (Z)-11-octadecen-1-yl acetate indicates that this compound is a sex pheromone for this species. The strong attraction in field bioassays, coupled with the lack of any other significant responses from antennae of male beetles in GC-EAD analyses of aeration extracts of females, strongly suggests that the pheromone consists of only this single component. Female-produced pheromones also have been found in species of the cerambycid subfamily Prioninae (Rodstein et al., 2011), and species that apparently are related to the Cerambycidae, including Migdolus fryanus Westwood (Anoplodermatinae; Leal et al., 1994) and Vesperus xatarti Dufour (Vesperidae; Boyer et al., 1997; for phylogenetic relationships, see Švácha and Danilevsky, 1986; Napp, 1994). Females of those species generally are larger than males and sedentary, being incapable of flight until after they have oviposited, permanently incapable of flight, or at least characteristically reluctant to fly (Linsley, 1959). The sedentary behavior of the females is consistent with the persistent nature of the larval hosts: living roots of perennial grasses, shrubs, or trees (e.g., Leal et al., 1994; Barbour et al., 2006). Individual hosts, therefore, can support many generations, and females need not disperse to locate appropriate new hosts (Hanks, 1999). Newly eclosed females of these species may remain concealed in the soil, or within tissues of the larval host, and release pheromones that attract their mates (Leal et al., 1994; Hanks, 1999; Barbour et al., 2006). The early release of pheromone by females, and strong response of males, suggests an urgency that is consistent with limited energy reserves due to the fact that the adults likely do not feed (Linsley, 1959; Hanks, 1999; Barbour et al., 2006).
In contrast to other species that have female-produced pheromones, the larvae of O. valida develop in hosts that are dying, or have recently died. Such hosts are unpredictable and ephemeral resources, since their tissues degrade rapidly (reviewed by Hanks, 1999). Therefore, adult females must be capable of dispersing to locate appropriate hosts, which is consistent with reports that they take flight readily (Linsley and Chemsak, 1976). This relationship between larval host condition and dispersal ability in adults is more consistent with species in the subfamilies Cerambycinae, Lamiinae, and Spondylidinae in which males produce pheromones that attract both sexes (Silk et al., 2007; Millar et al., 2009; Ray et al., 2009; Fonseca et al., 2010; Pajares et al., 2010). Larvae of those species also develop in highly stressed or moribund woody plants, and the pheromones apparently serve to expedite location of hosts (Hanks, 1999).
The structure of the O. valida pheromone is unprecedented among the cerambycid pheromones that have been reported to date, and completely unlike the diol/hydroxyketone motif of the volatile pheromones produced by species in the subfamily Cerambycinae (Millar et al., 2009), the terpene motif of the pheromones produced by males of species in the subfamily Spondylidinae (Silk et al., 2007), or the carboxylic acid motif of species in the genus Prionus (subfamily Prioninae; Rodstein et al., 2011). (Z)-11-Octadecen-1-yl acetate also is an aggregation pheromone produced by male fruit flies (Drosophila spp.; Bartelt et al., 1985), but when transferred to females during mating it subsequently discourages courtship of other males (Jallon et al., 1981). This compound also has been reported as a minor component in the defensive secretions of larvae of the chrysomelid beetle Linaeidea aenea (L.) (Sugawara et al., 1979). In addition, (Z)-11-octadecen-1-yl acetate is similar in structure to the monounsaturated acetate compounds that are pheromone components of many lepidopterans (El-Sayed, 2011).
References
Attygalle, A. B. 1998. Microchemical techniques, pp. 207–294, in J. G. Millar and K. F. Haynes (eds.) Methods in Chemical Ecology, Vol. 1. Chemical Methods. Chapman and Hall/Kluwer Academic Publishers, Norwell MA.
Barbour, J. D., Cervantes, D. E., Lacey, E. S., and Hanks, L. M. 2006. Calling behavior in the primitive longhorned beetle Prionus californicus Mots. J. Insect Behav. 19:623–629.
Bartelt, R. J., Schaner, A. M., and Jackson, L. L. 1985. Cis-vaccenyl acetate as an aggregation pheromone in Drosophila melanogaster. J. Chem. Ecol. 11:1744–1756.
Boyer, F. D., Malosse, C., Zagatti, P., and Einhorn, J. 1997. Identification and synthesis of vesperal, the female sex pheromone of the longhorn beetle Vesperus xatarti. Bull. Soc. Chim. Fr. 134:757–764.
Cope, J. 1984. Notes on the ecology of western Cerambycidae. Coleopts. Bull. 38:27–36.
Craighead, F. C. 1923. North American Cerambycid Larvae. Dominion of Canada, Department of Agriculture, Bull. 27, New Series (Technical).
El-Sayed, A. M. 2011. The Pherobase: Database of Insect Pheromones and Semiochemicals. <http://www.pherobase.com>
Fonseca, M. G., Vidal, D. M., and Zarbin, P. H. G. 2010. Male-produced sex pheromone of the cerambycid beetle Hedypathes betulinus: Chemical identification and biological activity. J. Chem. Ecol. 36:1132–1139.
Graham, E. E., Mitchell, R. F., Reagel, P. F., Barbour, J. D., Millar, J. G., and Hanks, L. M. 2010. Treating panel traps with a fluoropolymer enhances their efficiency in capturing cerambycid beetles. J. Econ. Entomol. 103:641–647.
Hanks, L. M. 1999. Influence of the larval host plant on reproductive strategies of cerambycid beetles. Annu. Rev. Entomol. 44:483–505.
Jallon, J. M., Antony, C., and Benamar, O. 1981. Un anti-aphrodisiaque produit par les males de Drosophila melanogaster et transféré aux femelles lors de la copulation. Comptes Rendues de l’Academie des Sciences, Paris 292:1147–1149.
Leal, W. S., Bento, J. M. S., Vilela, E. F., and Della Lucia, T. M. C. 1994. Female sex pheromone of the longhorn beetle Migdolus fryanus Westwood: N-(2’ S)-methylbutanoyl 2- ethylbutylamine. Experientia 50:853–856.
Linsley, E. G. 1959. Ecology of Cerambycidae. Annu. Rev. Entomol. 4:99–138.
Linsley, E. G. 1961. The Cerambycidae of North America: Part I. Introduction. Univ. Calif. Publ. Entomol. 18:1–135.
Linsley, E. G., and Chemsak, J. A. 1972. The Cerambycidae of North America: Part VI, No. 1. Taxonomy and classification of the subfamily Lepturinae. Univ. Calif. Publ. Entomol. 69:1–138.
Linsley, E. G., and Chemsak, J. A. 1976. The Cerambycidae of North America: Part VI, No. 2. Taxonomy and classification of the subfamily Lepturinae. Univ. Calif. Publ. Entomol. 80:1–186.
MacRae, T. C., and Rice, M. E. 2007. Biological and distributional observations on North American Cerambycidae (Coleoptera). Coleopt. Bull. 61:227–263.
Michelsen, A. 1963. Observations on the sexual behaviour of some longicorn beetles, subfamily Lepturinae (Coleoptera, Cerambycidae). Behaviour 22:152–166.
Millar, J. G., Hanks, L. M., Moreira, J. A., Barbour, J. D., and Lacey, E. S. 2009. Pheromone chemistry of cerambycid beetles, pp. 52–79, in K. Nakamura and J. G. Millar (eds.), Chemical Ecology of Wood-Boring Insects. Forestry and Forest Products Research Institute, Ibaraki, Japan.
Napp, D. S. 1994. Phylogenetic relationships among the subfamilies of Cerambycidae (Coleoptera–Chrysomeloidea). Rev. Bras. Entomol. 38:265–419.
Pajares, J. A., Álvarez, G., Ibeas, F., Gallego, D., Hall, D. R., and Farman, D. I. 2010. Identification and field activity of a male-produced aggregation pheromone in the pine sawyer beetle, Monochamus galloprovincialis. J. Chem. Ecol. 36:570–583.
Ray, A. M. 2009. Evolution and taxonomic distribution of volatile pheromones in cerambycine longhorned beetles. Doctoral dissertation, University of Illinois at Urbana-Champaign, Urbana, Illinois, USA. 111 p.
Ray, A. M., Millar, J. G., McElfresh, J. S., Swift, I. P., Barbour, J. D., and Hanks, L. M. 2009. Male-produced aggregation pheromone of the cerambycid beetle Rosalia funebris. J. Chem. Ecol. 35:96–103.
Rodstein, J., Millar, J. G., Barbour, J. D., McElfresh, J. S., Wright, I. M., Barbour, K. S., Ray, A. M., and Hanks, L. M. 2011. Determination of the relative and absolute configurations of the female-produced sex pheromone of the cerambycid beetle Prionus californicus. J. Chem. Ecol. (in press)
SAS Institute. 1999. SAS/STAT User’s Guide, 1st edition. SAS Institute Inc., Cary, NC.
Silk, P. J., Sweeney, J., Wu, J., Price, J., Gutowski, J. M., and Kerrela, E. G. 2007. Evidence for a male-produced pheromone in Tetropium fuscum (F.) and Tetropium cinnamopterum (Kirby) (Coleoptera: Cerambycidae). Naturwissenschaften 94:697–701.
Sokal, R. R. and F. J. Rohlf. 1995. Biometry, 3rd ed. W. H. Freeman, NY.
Sugawara, F., Matsuda, K., Kobayashi, A., and Yamashita, K. 1979. Defensive secretion of chrysomelid larvae Linaeidea aenea Linné and Plagiodera versicolora distincta Baly. J. Chem. Ecol. 5:929–938.
Švácha, P., and Danilevsky, M. 1986. Cerambycoid larvae of Europe and Soviet Union (Coleoptera: Cerambycoidea). Part I. Acta U. Carol. Biol. 30:1–176.
Švácha, P., and Danilevsky, M. 1988. Cerambycoid larvae of Europe and Soviet Union (Coleoptera: Cerambycoidea). Part III. Acta U. Carol. Biol. 32:1–205.
Swaine, J. M., and Hopping, R. 1928. The Lepturini of America North of Mexico. Part I. Can. Nat. Mus. Bull. 52:1–97.
Vig, O. P., Sharma, M. L., Sabharwal, A., Rashmi, R., and Huq, M. A. 1988. A new synthesis of cis-vaccenyl acetate and vaccenaldehyde. J. Indian Chem. Soc. 65:671–672.
Acknowledgments
We thank Tom Coleman, USDA Forest Service, for information about field sites and for support of this work, Ian Swift for discussions on taxonomy and natural history, assistance with the literature, and for comments on an early version of this work, and Rebeccah Waterworth for assistance with field work and GC-EAD. We are grateful for financial support from the Alphawood Foundation (to LMH), and the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service, grant number #2009-35302-05047 (to JGM and LMH).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Ray, A.M., Žunič, A., Alten, R.L. et al. cis-Vaccenyl Acetate, A Female-Produced Sex Pheromone Component of Ortholeptura valida, A Longhorned Beetle in the Subfamily Lepturinae. J Chem Ecol 37, 173–178 (2011). https://doi.org/10.1007/s10886-011-9908-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-011-9908-5