Abstract
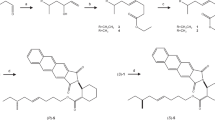
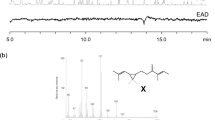
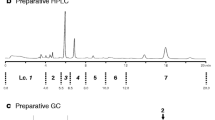
The absolute configuration of the sex pheromone of the citrophilous mealybug, Pseudococcus calceolariae, was determined to be (1R,3R)-[2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropyl]methyl (R)-2-acetoxy-3-methylbutanoate. NMR, derivatization reactions, chiral gas chromatography-mass spectrometry, and comparison with synthetic chiral reference compounds, were used to determine the absolute configuration of this compound. This activity of this compound was further confirmed by testing synthetic stereoisomers of the compound as lures in traps for adult male mealybugs. Traps baited with 1,000 μg of the pheromone compound caught 36 times more males than traps baited with virgin females. A mixture of stereoisomers of the pheromone compound can be used for field trapping without adverse effects on trap catch. A comparison with the structures of other sex pheromones of mealybugs is presented.






Similar content being viewed by others
References
Arai, T., Sugie, H., Hiradate, S., Kuwahara, S., Itagaki, N., and Nakahata, T. 2003. Identification of a sex pheromone component of Pseudococcus cryptus. J. Chem. Ecol. 29:2213–2223.
Bartlett, B. R. 1978. Pseudococcidae. pp. 137–170 in C. P. Clausen (ed.). Introduced Parasites and Predators of Arthropod Pests and Weeds: A World Review. Agric Res. Service, Agriculture Handbook nr 480, U.S. Dep. Agric., Washington DC.
Bierl-Leonhardt, B. A., Moreno, D. S., Schwarz, M., Fargerlund, J., and Plimmer, J. R. 1981. Isolation, identification and synthesis of the sex pheromone of the citrus mealybug, Planococcus citri (Risso). Tetrahedron Lett. 22:389–92.
Bramwell, A. F., Chrombie, L., Hemesley, P., Pattenden, G., Elliott, M., and Janes, N. F. 1969. Nuclear magnetic resonance spectra of the natural pyrethrins and related compounds. Tetrahedron 25:1727–1741.
El-Sayed, A. M. 2009. The Pherobase: Database of Insect Pheromones and Semiochemicals. http://www.pherobase.com.
El-Sayed, A. M., Unelius, C. R., Twidle, A., Mitchell, V., Manning, L.-A., Cole, L., Suckling, D. M., Flores, M. F., Zaviezo, T., and Bergmann, J., 2010. Chrysanthemyl 2-acetoxy-3-methylbutanoate: sex pheromone of the citrophilous mealybug, Pseudococcus calceolariae. Tetrahedron Lett. 51:1075–1078.
Figadere, B. A., Mcelfresh, J. S., Borchardt, D., Daane, K. M., Bentley, W., and Millar, J. G. 2007. Trans-alpha-necrodyl isobutyrate, the sex pheromone of the grape mealybug, Pseudococcus maritimus. Tetrahedron Lett. 48:8434–8437.
Hinkens, D. M., McElfresh, J. S., and Millar, J. G., 2001. Identification and synthesis of the sex pheromone of the vine mealybug, Planococcus ficus. Tetrahedron Lett. 42:1619–1621.
Ho, H.-Y., Hung, C.-C., Chuang, T.-H., and Wang, W. L. 2007. Identification and synthesis of the sex pheromone of the passionvine mealybug, Planococcus minor (Maskell). J. Chem. Ecol. 33:1986–1996.
Ho, H.-Y., Su, Y.-T., Ko, C.-H., and Tsai, M.-Y. 2009. Identification and synthesis of the sex pheromone of the Madeira mealybug, Phenacoccus madeirensis Green. J. Chem. Ecol. 35:724–732.
Millar, J. G., Daane, K. M., McElfresh, J. S., Moreira, J. A., and Bentley, W. J. 2005a. Chemistry and applications of mealybug sex pheromones, pp 11-27. In R. J. Petroski, M. R. Tellez, and R. W. Behle (eds). ACS Symposium Series 906, Semiochemicals in Pest and Weed Control. American Chemical Society, Washington, D.C.
Millar, J. G., Midland, S. L., McElfresh, J. S., and Daane, K. M. 2005b. (2,3,4,4-Tetramethylcyclopentyl)methyl acetate, a sex pheromone from the obscure mealybug: first example of a new structural class of monoterpenes. J. Chem. Ecol. 31:2999–3005.
Millar, J. G., Moreira, J. A., McElfresh, J. S., Daane, K. M., and Freund, A. S. 2009. Sex pheromone of the longtailed mealybug: a new class of monoterpene structure. Org. Lett. 11:2683–2685.
Nakahata, T., Itagaki, N., Arai, T., Sugie, H., and Kuwahara, S. 2003. Synthesis of the sex pheromone of the citrus mealybug, Pseudococcus cryptus. Biosci., Biotechnol., Biochem. 67:2627–2631.
Negishi, T., Uchida, M., Tamaki, Y., Mori, K., Ishiwatari, T., Asano, S., and Nakagawa, K. 1980. Sex pheromone of the comstock mealybug, Pseudococcus comstocki Kuwana: isolation and identification. Appl. Entomol. Zool., 15:328–33
Sugie, H., Teshiba, M., Narai, Y., Tsutsumi, T., Sawamura, N., Tabata, J., and Hiradate, S. 2008. Identification of a sex pheromone component of the Japanese mealybug, Planococcus kraunhiae (Kuwana). Appl. Entomol. Zool. 43:369–375.
Takano, S., Tanaka, M., Seo, K., Hirama, M., and Ogasawara, K. 1985. General chiral route to irregular monoterpenes via a common intermediate: syntheses of (S)-lavandulol, cis(1S,3R)-chrysanthemol, (1R,2S)-rothrockene, and (R)-santolinatriene. J. Org. Chem. 50:931–936.
Zhang, A. and Amalin, D. 2005. Sex pheromone of the pink hibiscus mealybug, Maconellicoccus hirsutus (Green) (Homoptera: Pseudococcidae): biological activity evaluation. Environ. Entomol. 34:264–270.
Zhang, A., Nie, J., and Khrimian, A. 2004a. Chiral synthesis of maconelliol: a novel cyclobutanoid terpene alcohol from pink hibiscus mealybug, Maconellicoccus hirsutus. Tetrahedron Lett. 45:9401–9403.
Zhang, A., Amalin, D., Shirali, S., Serrano, M. S., Franqui, R. A., Oliver, J. E., Klun, J. A., Aldrich, J. R., Meyerdirk, D. E., and Lapointe, S. L. 2004b. Sex pheromone of the pink hibiscus mealybug, Maconellicoccus hirsutus, contains an unusual cyclobutanoid monoterpene. Proc. Natl. Acad. Sci. U.S.A. 101:9601–9606.
Zhang, A., Wang, S., Vitullo, J., Roda, A., Mannion, C., and Bergh, J.C. 2006. Olfactory discrimination among sex pheromone stereoisomers: chirality recognition by pink hibiscus mealybug males. Chem. Senses 31:621–626.
Acknowledgements
This work was supported by the Foundation for Research Science and Technology, FRST (Sustainable Integrated Pest Management in Horticulture, C06X0811) and the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT grant 11060527). We thank Tom Sullivan (New Zealand) and Alda Romero (Chile) for maintaining mealybug colonies and Lyn Cole for assistance with the New Zealand field trials. Financial funding from the Linnaeus University, Kalmar, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Unelius, C.R., El-Sayed, A.M., Twidle, A. et al. The Absolute Configuration of the Sex Pheromone of the Citrophilous Mealybug, Pseudococcus calceolariae . J Chem Ecol 37, 166–172 (2011). https://doi.org/10.1007/s10886-010-9904-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-010-9904-1