Abstract
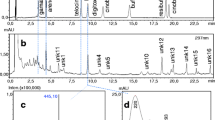
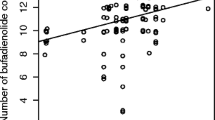
We conducted a quantitative and qualitative chemical analysis of cane toad bufadienolides—the cardioactive steroids that are believed to be the principal cane toad toxins. We found complex shifts in toxin composition through toad ontogeny: (1) eggs contain at least 28 dominant bufadienolides, 17 of which are not detected in any other ontogenetic stage; (2) tadpoles present a simpler chemical profile with two to eight dominant bufadienolides; and (3) toxin diversity decreases during tadpole life but increases again after metamorphosis (larger metamorph/juvenile toads display five major bufadienolides). Total bufadienolide concentrations are highest in eggs (2.64 ± 0.56 μmol/mg), decreasing during tadpole life stages (0.084 ± 0.060 μmol/mg) before rising again after metamorphosis (2.35 ± 0.45 μmol/mg). These variations in total bufadienolide levels correlate with toxicity to Australian frog species. For example, consumption of cane toad eggs killed tadpoles of two Australian frog species (Limnodynastes convexiusculus and Litoria rothii), whereas no tadpoles died after consuming late-stage cane toad tadpoles or small metamorphs. The high toxicity of toad eggs reflects components in the egg itself, not the surrounding jelly coat. Our results suggest a dramatic ontogenetic shift in the danger that toads pose to native predators, reflecting rapid changes in the types and amounts of toxins during toad development.




Similar content being viewed by others
References
Akimova, O. A., Bagrov, A. Y., Lopina, O. D., Kamernitsky, A. V., Tremblay, J., Hamet, P., and Orlov, S. N. 2005. Cardiotonic steroids differentially affect intracellular Na+ and [Na+] i /[K+] i -independent signalling in C7-MDCK cells. J. Biol. Chem. 280:802–819.
Akizawa, T., Mukai, T., Matsukawa, M., Yoshioka, M., Morris, J. F., and Butler, V. P. Jr. 1994. Structures of novel bufadienolides in the eggs of a toad, Bufo marinus. Chem. Pharmacol. Bull. 42:754–756.
Alford, R. A., Cohen, M. P., Crossland, M. R., Hearnden, M. N., James, D., and Schwarzkopf, L. 1995. Population biology of Bufo marinus in northern Australia, pp. 173–181, in M. Finlayson (ed.). Wetland Research in the Wet–Dry Tropics of Australia Office of the Supervising Scientist, Canberra Supervising Scientist Report Number 101.
Arnold, S. J., and Wassersug, R. J. 1978. Differential predation on metamorphic anurans by garter snakes (Thamnophis): social behavior as a possible defense. Ecology 59:1014–1022.
Brodie, E. D. Jr., Formanowicz, D. R. J, and 1987. Antipredator mechanisms of larval amphibians: protection of palatable individuals. Herpetologica 43:369–373.
Brodie, E. D. Jr., Formanowicz, D. R. Jr., and Brodie, E. D. III. 1978. The development of noxiousness of Bufo americanus tadpoles to aquatic insect predators. Herpetologica 34:302–306.
Burnett, S. 1997. Colonising cane toads cause population declines in native predators: reliable anecdotal information and management implications. Pacific Conserv. Biol. 3:65–72.
Child, T., Phillips, B. L., and Shine, R. 2008. Abiotic and biotic influences on the dispersal behaviour of metamorph cane toads (Bufo marinus) in tropical Australia. J. Exp. Zool. 309A:215–224.
Cohen, M. P., and Alford, R. A. 1993. Growth, survival, and activity patterns of Bufo marinus metamorphs. Wildl. Res. 20:1–13.
Crossland, M. 1998. Ontogenetic variation in the toxicity of tadpoles of the introduced cane toad Bufo marinus to native Australian aquatic invertebrate predators. Herpetologica 54:364–369.
Crossland, M. R., and Alford, R. A. 1998. Evaluation of the toxicity of eggs, hatchlings and tadpoles of the introduced toad Bufo marinus (Anura, Bufonidae) to native Australian aquatic predators. Aust. J. Ecol. 23:129–137.
Crossland, M. R., and Azevedo-Ramos, C. 1999. Effects of Bufo (Anura: Bufonidae) toxins on tadpoles from native and exotic Bufo habitats. Herpetologica 55:192–199.
Crossland, M. R., Brown, G. P., Anstis, M., Shilton, C. M., and Shine, R. 2008. Mass mortality of native anuran tadpoles in tropical Australia due to the invasive cane toad (Bufo marinus). Biol. Conserv. 141:2387–2394.
Daly, J. W., Garraffo, H. M., Spande, T. F., Giddings, L. -A., Saporito, R. A., Vieites, D. R., and Vences, M. 2008. Individual and geographic variation of skin alkaloids in three species of Madagascan poison frogs (Mantella). J Chem. Ecol. 34:252–259.
Doody, J. S., Green, B., Sims, R., Rhind, D., West, P., and Steer, D. 2006. Indirect impacts of invasive cane toads (Bufo marinus) on nest predation in pig-nosed turtles (Carettochelys insculpta). Wildl. Res. 33:349–354.
Duellman, W. E., and Trueb, L. 1986. Biology of amphibians. McGraw-Hill, New York.
Frost, D. R., Grant, T., Faivovich, J., Bain, R. H., Haas, A., Haddad, C. F. B., De Sa, R. O., Channing, A., Wilkinson, M., Donnellan, S. C., Raxworthy, C. J., Campbell, J. A., Blotto, B. L., Moler, P., Drewes, R. C., Nussbaum, R. A., Lynch, J. D., Green, D. M., and Wheeler, W. C. 2006. The amphibian tree of life. Bull. Am. Mus. Nat. Hist. 297:1–370.
Gosner, K. L. 1960. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190.
Griffiths, A. D., and McKay, J. L. 2007. Cane toads reduce the abundance and site occupancy of Merten’s water monitor (Varanus mertensi). Wildl. Res. 34:609–615.
Grubb, J. D. 1972. Differential predation by Gambusia affinis on the eggs of seven species of anuran amphibians. Am. Midl. Nat. 88:102–108.
Gunzburger, M. S., and Travis, J. 2005. Critical literature review of the evidence for unpalatability of amphibian eggs and larvae. J. Herpetol. 39:547–571.
Hanifin, C. T., Brodie, E. D. III., and Brodie, E. D. II. 2004. Tetrodotoxin levels in eggs of the rough-skin newt, Taricha granulosa, are correlated with female toxicity. J. Chem. Ecol. 29:1729–1739.
Heyer, R. W., McDiarmid, R. W., and Weigmann, D. L. 1975. Tadpoles, predation and pond habitats in the tropics. Biotropica 7:100–111.
Jennings, W. S. Jr., and Schaefer, G. C. 1978. Antipredative function of the gelatinous coating of the eggs of the frog, Rana p. pipiens Schreber (Amphibia: Ranidae). Virginia J. Sci. 29:62.
Keenan, S. M., Delisle, R. K., Welsh, W. J., Paula, S., and Ball, W. J. 2005. Elucidation of the Na+, K+-ATPase digitalis binding site. J. Mol. Graph. Model. 23:465–475.
Kelehear, C. 2007. The effects of lung nematodes (Rhabdias cf. hylae) on metamorph cane toads (Chaunus marinus), and implications for biological control. B.Sc. (Honours) thesis, School of Biological Sciences, University of Sydney.
Lawler, K. L., and Hero, J. M. 1997. Palatability of Bufo marinus tadpoles to a predatory fish decreases with development. Wildl. Res. 24:327–334.
Letnic, M., Webb, J. K., and Shine, R. 2008. Invasive cane toads (Bufo marinus) cause mass mortality of freshwater crocodiles (Crocodylus johnstoni) in tropical Australia. Biol. Conserv. 141:1773–1782.
Lever, C. 2001. The cane toad. The history and ecology of a successful colonist. Westbury Academic, Otley.
Licht, L. E. 1968. Unpalatability and toxicity of toad eggs. Herpetologica 24:93–98.
Mack, R. N., Simberloff, D., Lonsdale, W. M., Evans, H., Clout, M., and Bazzaz, F. 2000. Biotic invasions: causes, epidemiology, global consequences and control. Ecol. Appl. 10:689–710.
Mahony, M., and Clulow, J. 2006. Control of cane toads by sterile male release and inherited sterility pp. 134–150, in K. L. Molloy, and W. R. Henderson (eds.). Proceedings of the Cane Toad Workshop, CRC for Invasive Animals, Brisbane, June 2006. Invasive Animals Cooperative Research Centre, Canberra.
Matsukawa, M., Akizawa, T., Mukai, T., Yoshioka, M., Morris, J. F., and Butler, V. P. Jr. 1994. Structures and biological activities of bufadienolides from the toad, Bufo marinus. Tennen Yuki Kagobutsu Toronkai Koen Yoshishu 36:807–814.
Mooney, H. A., and Cleland, E. E. 2001. The evolutionary impact of invasive species. Proc. Nat. Acad. Sci. U. S. A. 98:5446–5451.
Okimura, H., Yassuhara, J. C., Fambrough, D. M., and Takeyasu, K. 2002. P-type ATPases in Caenorhabditis and Drosophila: implications for evolution of the P-type ATPase subunit families with special reference to the Na, K-ATPase and H, K-ATPase subgroup. J. Membr. Biol. 191:13–24.
Pallister, J., Voysey, R., Olsen, V., and Hyatt, A. 2006. Viral delivery of cane toad biological control pp. 89–93, in K. L. Molloy, and W. R. Henderson (eds.). Proceedings of the Cane Toad Workshop, CRC for Invasive Animals, Brisbane, June 2006 Invasive Animals Cooperative Research Centre, Canberra.
Peterson, J. A., and Blaustein, A. R. 1992. Relative palatabilities of anuran larvae to natural aquatic predators. Copeia 1992:577–584.
Phillips, B. L., Brown, G. P., and Shine, R. 2003. Assessing the potential impact of cane toads on Australian snakes. Conserv. Biol. 17:1738–1747.
Phisalix, C. 1903. Corrélations fonctionnelles entre les glandes à venin et l’ovaire chez le Crapaud commun. C. R. Acad. Sci. 137:1082–1084.
Phisalix, M. 1922. Animaux Venimeux et Venins, vol. 2. Masson et Cie, Paris.
Pramuk, J. B. 2006. Phylogeny of South American Bufo (Anura: Bufonidae) inferred from combined evidence. Zool. J. Linn. Soc. 146:407–452.
Pramuk, J. B., Robertson, T., Sites, J. W. Jr., and Noonan, B. P. 2008. Around the world in 10 million years: biogeography of the nearly cosmopolitan true toads (Anura: Bufonidae). Global Ecol. Biogeog. 17:72–83.
Sandlund, O. T., Schei, P. J., and Viken, A. 1999. Invasive species and biodiversity management. Kluwer Academic, Boston.
Schlaepfer, M. A., Runge, M. C., and Sherman, P. W. 2002. Ecological and evolutionary traps. Trends Ecol. Evol. 17:474–480.
Shine, R., Brown, G. P., Phillips, B. L., Webb, J. K., and Hagman, M. 2006. The biology, impact and control of cane toads: an overview of the University of Sydney’s research program, pp. 18–32, in K. L. Molloy, and W. R. Henderson (eds.). Proceedings of the Cane Toad Workshop, CRC for Invasive Animals, Brisbane, June 2006 Invasive Animals Cooperative Research Centre, Canberra.
Steyn, P. S., and Van Heerden, F. R. 1998. Bufadienolides of plant and animal origin. Nat. Prod. Rep. 15:397–413.
Wallick, E. T., and Schwartz, A. 1988. Interaction of cardiac glycosides with Na+, K+-ATPase. Meth. Enzymol. 156:201–213.
Ward, D., and Sexton, O. J. 1981. Anti-predator role of salamander egg membranes. Copeia 1981:724–726.
Werschkul, D. F., and Christensen, M. T. 1977. Differential predation by Lepomis macrochirus on the eggs and tadpoles of Rana. Herpetologica 33:237–241.
Acknowledgements
We thank the Australian Research Council, Queensland State Government, and Invasive Animals Cooperative Research Center for funding and David Nelson for assistance with bioassay experiments. Procedures involving live animals were approved by the University of Sydney Animal Care and Ethics Committee.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
Total amount of each bufadienolide detected (μmol) in each life history stage of the cane toad. Bufadienolides are listed by retention time (RT). All data are shown as mean ± SEM. “−” means below detectable levels. The “metamorph” category refers to small metamorphs (<13 mm SUL), whereas the “juveniles” category refers to metamorphs >16 mm SUL (DOC 72.0 KB)
Rights and permissions
About this article
Cite this article
Hayes, R.A., Crossland, M.R., Hagman, M. et al. Ontogenetic Variation in the Chemical Defenses of Cane Toads (Bufo marinus): Toxin Profiles and Effects on Predators. J Chem Ecol 35, 391–399 (2009). https://doi.org/10.1007/s10886-009-9608-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-009-9608-6