Abstract
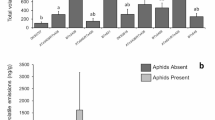
The profiles of volatile chemicals emitted by Vicia faba plants damaged by Lygus rugulipennis feeding, and by feeding plus oviposition, were shown to be quantitatively different from those released by undamaged plants. Samples of volatile chemicals collected from healthy plants, plants damaged by males as a consequence of feeding, plants damaged by females as a consequence of feeding and oviposition, plants damaged by feeding with mated males still present, and plants damaged by feeding and oviposition with gravid females still present, showed significant differences in the emission of hexyl acetate, (Z)-β-ocimene, (E)-β-ocimene, (E)-β-caryophyllene, and methyl salicylate. In particular, treatments with mated females present on plants had a significant increase in emission levels of the above compounds, possibly due to eggs laid within plant tissues or active feeding, compared with undamaged plants and plants damaged by males feeding, with or without insects still present. Furthermore, the pheromonal blend released by mated L. rugulipennis females, mainly comprising hexyl butyrate, (E)-2-hexenyl butyrate, and (E)-4-oxo-2-hexenal, was enhanced when females were active on broad bean plants, whereas such an increase was not observed in males. Both sexes gave electroantennogram responses to green leaf volatiles from undamaged plants and to methyl salicylate and (E)-β-caryophyllene emitted by Lygus-damaged plants, suggesting that these compounds may be involved in colonization of host plants by L. rugulipennis. In addition, mated males and females were responsive to hexyl butyrate, (E)-2-hexenyl butyrate, and (E)-4-oxo-2-hexenal released by mated females on V. faba, indicating that these substances could have a dual function as a possible aggregation pheromone in female–female communication, and as a sex pheromone in female–male communication.



Similar content being viewed by others
References
Bernays, E. A., and Chapman, R. F. 1994. Host-Plant Selection by Phytophagous Insects. Chapman & Hall, London, UK.
Birkett, M. A., Chamberlain, K., Guerrieri, E., Pickett, J. A., Wadhams, L. J., and Yasuda, T. 2003. Volatiles from whitefly-infested plants elicit a host-locating response in the parasitoid, Encarsia formosa. J. Chem. Ecol 29:1589–1600.
Blackmer, J. L., Rodriguez-Saona, C., Byers, J. A., Shope, K. L., Smith, J. P. 2004. Behavioral response of Lygus hesperus to conspecifics and headspace volatiles of alfalfa in a Y-tube olfactometer. J. Chem. Ecol 30:1547–1564.
Chamberlain, K., Guerrieri, E., Pennacchio, F., Pettersson, J., Pickett, J. A., Poppy, G. M., Powell, W., Wadhams, L. J., Woodcock, C. M. 2001. Can aphid-induced plant signals be transmitted aerially and through the rhizosphere. Biochem. Syst. Ecol 29:1063–1074.
Colazza, S., Fucarino, A., Peri, E., Salerno, G., Conti, E., and Bin, F. 2004a. Insect oviposition induces volatile emission in herbaceous plants that attracts egg parasitoids. J. Exp. Biol 207:47–53.
Colazza, S., Mcelfresh, J. S., and Millar, J. G. 2004b. Identification of volatiles synomones, induced by Nezara viridula feeding and oviposition on bean spp., that attract the egg parasitoid Trissolcus basalis. J. Chem. Ecol. 30:945–963.
Conti, E., Zadra, Z., Salerno, G., Leombruni, B., Volpe, D., Frati, F., Marucchini, C., and Bin, F. 2008. Changes in the volatile profile of Brassica oleracea due to feeding and oviposition by Murgantia histrionica (Heteroptera: Pentatomidae). Eur. J. Entomol 105:839–847.
Cook, S. M., Rasmussen, H. B., Birkett, M. A., Murray, D. A., Pye, B. J., Watts, N. P., and Williams, I. H. 2007. Behavioural and chemical ecology underlying the success of turnip rape (Brassica rapus) trap crops in protecting oilseed rape (Brassica napus) from the pollen beetle (Meligethes aeneus). Arthropod-Plant Interact 1:57–67.
De Moraes, C. M., Lewis, W. J., Paré, P. W., Alborn, H. T., and Tumlinson, J. H. 1998. Herbivore-infested plants selectively attract parasitoids. Nature 393:570–573.
Deng, J.-Y., Wei, H.-Y., Huang, Y.-P., and Du, J.-W. 2004. Enhancement of attraction to sex pheromones of Spotoptera exigua by volatile compounds produced by host plants. J. Chem. Ecol 30:2037–2045.
Dicke, M., and van Loon, J. J. A. 2000. Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context. Entomol. Exp. Appl 97:237–249.
Du, Y., Poppy, G. M., Powell, W., Pickett, J. A., Wadhams, L. J., and Woodcock, M. C. 1998. Identification of semiochemicals released during aphid feeding that attract parasitoid Aphidius ervi. J. Chem. Ecol 24:1355–1368.
Frati, F., Salerno, G., Conti, E., and Bin, F. 2008. Role of the plant-conspecific complex in host location and intra-specific communication of Lygus rugulipennis. Physiol. Entomol 33:129–137.
Gaoni, Y. 1977. Lithium aluminium hydride promoted extrusion of SO2 from sulfolenes and sulfolene-adducts. 1,3-Dienes, 3-chloro-and 3,3-dichloro-1,4-dienes and skipped polyenes. Tetrahedron Lett 11:947–950.
Glinwood, R., Pettersson, J., Kularatne, S., Ahmed, E., and Kumar, V. 2003. Female European tarnished plant bugs, Lygus rugulipennis (Heteroptera: Miridae), are attracted to odours from conspecific females. Acta Agric. Scand. A Anim. Sci. 53:29–32.
Guerrieri, E., Poppy, G. M., Powell, W., Rao, R., and Pennacchio, F. 2002. Plant-to-plant communication mediating in-flight orientation of Aphidius ervi. J. Chem. Ecol 28:1703–1714.
Ho, H., and Millar, J. G. 2002. Identification, electroantennogram screening, and field bioassays of volatile chemicals from Lygus hesperus Knight (Heteroptera: Miridae). Zool. Stud 41:311–320.
Holopainen, J. K., and Varis, A.-L. 1991. Host plants of the European tarnished plant bug Lygus rugulipennis Popp. (Heteroptera: Miridae). J. Appl. Entomol 111:484–498.
Innocenzi, P. J., Hall, D. R., Cross, J. V., Masuh, H., Phythian, S. J., Chittamaru, S., and Guarino, S. 2004. Investigation of long-range female sex pheromone of the European tarnished plant bug, Lygus rugulipennis: chemical, electrophysiological and field studies. J. Chem. Ecol 30:1509–1529.
Innocenzi, P. J., Hall, D. R., Cross, J. V., and Hesketh, H. 2005. Attraction of male European tarnished plant bug, Lygus rugulipennis to components of the female sex pheromone in the field. J. Chem. Ecol 31:1401–1413.
Kessler, A., and Baldwin, I. T. 2001. Defensive function of herbivore-induced plant volatile emissions in nature. Science 291:2141–2144.
Landolt, P. J., Heath, R. R., Millar, J., Davis-Hernandez, K. M., Dueben, B. D., and Ward, K. E. 1994. Effects of host plant, Gossypium hirsutum L., on sexual attraction of cabbage looper moths, Trichoplusia ni (Hübner) (Lepidoptera: Noctuidae). J. Chem. Ecol 20:2959–2974.
Maddrell, S. H. P. 1969. Secretion by the Malphigian tubules of Rhodnius. The movement of ions and water. J. Exp. Biol. 51:71–97.
Mcneil, J. N., and Delisle, J. 1989. Are host plants important in pheromone-mediated mating systems of Lepidoptera. Experientia 45:236–240.
Meiners, T., and Hilker, M. 2000. Induction of plant synomones by oviposition of a phytophagous insect. J. Chem. Ecol 26:221–232.
Moreira, J. A., and Millar, J. 2005. Short and simple syntheses of 4-oxo-(E)-2-hexenal and homologues: pheromone components and defensive compounds of Hemiptera. J. Chem. Ecol 31:965–968.
Pickett, J. A. 1990. GC–MS in insect pheromone identification: three extreme case histories, pp. 299–309, in A. R. McCaffery, and I. D. Wilson (eds.). Chromatography and Isolation of Insect Hormones and PheromonesPlenum, New York.
Price, P. W. 1997. Insect ecology. John Wiley & Son, Inc., New York, USA.
Prosser, I., Altug, I. G., Philllips, A. L., Konig, W. A., Bouwmeester, H. J., and Beale, M. H. 2004. Enantiospecific (+) and (−)-germacrene D synthases, cloned from goldenrod, reveal a functionally active variant of the universal isoprenoid-biosynthesis aspartate-rich motif. Arch. Biochem, Biophys 432:136–144.
Rodriguez-Saona, C., Crafts-Brandner, S. J., Williams, L. III, and Paré, P. W. 2002. Lygus hesperus feeding and salivary gland extracts induce volatile emissions in plants. J. Chem. Ecol 28:1733–1747.
STATSOFT INC 2001. Statistica (data analysis software system), version 6. StatSoft Italia S.r.l., Vigonza (PD), Italy.
Turlings, T. C. J., Lengwiler, U. B., Bernasconi, M. L., and Wechsler, D. 1998. Timing of induced volatile emissions in maize seedlings. Planta 207:146–152.
Visser, J. H. 1986. Host odor perception in phytophagous insects. Annu. Rev. Entomol 31:121–144.
Wadhams, L. J. 1990. The use of coupled gas chromatography: electrophysiological techniques in the identification of insect pheromones, pp. 289–298, in A. R. McCaffery, and I.D. Wilson (eds.). Chromatography and Isolation of Insect Hormones and PheromonesPlenum, New York.
Wegener, R., Schulz, S., Meiners, T., Hadwich, K., and Hilker, M. 2001. Analyses of volatiles induced by oviposition of the elm leaf beetle Xanthogaleruca luteola on Ulmus minor. J. Chem. Ecol 27:499–515.
Yang, Z., Bengtsson, M., and Witzgall, P. 2003. Host plant volatiles synergize response to sex pheromone in codling moth, Cydia pomonella. J. Chem. Ecol 30:619–629.
Zar, J. H. 1999. Biostatistical Analysis. Prentice Hall, NJ, USA.
Acknowledgments
We thank Laura Chiattelli and Donatella Marchionni for rearing Lygus bugs and Keith Plumb for growing Vicia faba plants. We also are grateful to the two anonymous reviewers and the editor for thoughtful comments. This research was financially supported by the Ministry for University and Scientific Technological Research (PRIN 2002 “Functional investigations on trophic webs, plants-Hemiptera-parasitoids, and biological control”). The authors have contributed equally to this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s10886-009-9625-5
Rights and permissions
About this article
Cite this article
Frati, F., Chamberlain, K., Birkett, M. et al. Vicia faba–Lygus rugulipennis Interactions: Induced Plant Volatiles and Sex Pheromone Enhancement. J Chem Ecol 35, 201–208 (2009). https://doi.org/10.1007/s10886-008-9572-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-008-9572-6