Abstract
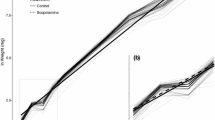
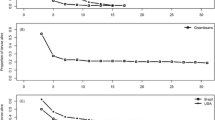
Brugmansia suaveolens (Solanaceae) contains tropane alkaloids (TAs), which can act as chemical defenses. Selective pressures might modulate the allocation of alkaloids within the plant, as postulated by optimal-defense theory. By tracing scopolamine, the most abundant TA in this species, we found that scopolamine in an artificial diet, in concentrations similar to those in leaves of B. suaveolens, increased mortality and prolonged developmental time of the larvae of the generalist noctuid moth Spodoptera frugiperda. A diet of undamaged leaves of B. suaveolens also showed a large negative effect on the growth of larvae of S. frugiperda compared to a diet of leaves of Ricinus communis, a species that did not have negative effects on this moth; more valuable plant parts, such as young leaves, flowers, and unripe fruits with seeds, have higher scopolamine concentrations than other tissues; leaves of B. suaveolens increase their content of scopolamine after artificial damage. The highest induction was found 24 hr after the damage, and after that, scopolamine content decreased to constitutive levels. This increase represented a cost, because in another experiment, a treatment with methyl jasmonate, an elicitor hormone, increased scopolamine production 9.5-fold and decreased leaf growth 2.3-fold; a diet of artificially damaged leaves of B. suaveolens showed a negative effect on the growth of larvae of S. furgiperda compared to undamaged leaves, suggesting that damage by herbivores induces resistance. Our data are in line with the optimal-defense theory, but experiments in the field with herbivores that share an evolutionary history with B. suaveolens must be undertaken to understand the dynamics of TA allocation in response to herbivory.




Similar content being viewed by others
References
Agrawal, A. A. 1998. Leaf damage and associated cues induce aggressive ant recruitment in a neotropical ant-plant. Ecology 79:2100–2112.
Baldwin, I. T. 1998. Jasmonate-induced responses are costly but benefit plants under attack in native populations. Proc. Natl. Acad. Sci. U. S. A. 95:8113–8118.
Berenbaum, M. R. 1983. Coumarins and caterpillars: a case for coevolution. Evolution 37:163–179.
Blau, P. A., Feeny, P., Contardo, L., and Robson, D. S. 1978. Allylglucosinolate and herbivore caterpillars: a contrast in toxicity and tolerance. Science 200:1296–1298.
Bultman, T. L. and Bell, G. D. 2003. Interaction between fungal endophytes and environmental stressors influences plant resistance to insects. Oikos 103:182–190.
Choi, Y. H., Chin, Y.-W., Kim, J., Jeon, S. H., and Yoo, K.-P. 1999. Strategies for supercritical fluid extraction of hyoscyamine and scopolamine salts using basified modifiers. J. Chromatogr. A 863:47–55.
Cipollini, M. L. 2000. Secondary metabolites of vertebrate-dispersed fruits: Evidence for adaptive functions. Rev. Chil. Hist. Nat. 73:421–440.
Cipollini, M. L. and Levey, D. J. 1997. Secondary metabolites of fleshy vertebrate-dispersed fruits: Adaptive hypotheses and implications for seed dispersal. Am. Nat. 150:346–372.
Croteau, R., Kutchan, T. M., and Lewis, G. L. 2000. Natural products, pp. 1250–1317, in B. Buchanan, W. Gruissem, and R. Jones (eds.). Biochemistry & Molecular Biology of Plants, vol 24. American Society of Plant Physiologists, Rockville, MD.
Dicke, M. and van Loon, J. J. A. 2000. Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context. Entomol. Exp. Appl. 97:237–249.
Dyer, L. A., Dodson, C. D., Stireman III, J. O., Tobler, M. A., Smilanich, A. M., Fincher, R. M., and Letourneau, D. K. 2003. Synergistic effects of three Piper amides on generalist and specialist herbivores. J. Chem. Ecol. 29:2499–2514.
Edelstein-Keshet, L. and Rausher, M. 1989. The effects of inducible plant defenses on herbivory populations. I. Mobile herbivores in continuous time. Am. Nat. 133:787–810.
Ferraz, J. M. G. 1991. Estudos bioecológicos sobre Spodoptera frugiperda (Abbot e Smith, 1797) (Lepidoptera: Noctuidae) como subsídio ao manejo integrado de pragas na cultura do milho. Ph.D. Thesis. Universidade Estadual de Campinas, São Paulo.
Freitas, A. V. L. 1993. Biology and population dynamics of Placidula euryanassa (Felder), a relict ithomiine butterfly (Lepidoptera: Ithomiinae). J. Lepid. Soc. 47:87–105.
Freitas, A. V. L., Trigo, J. R., Brown, K. S., Witte, L., Hartmann, T., and Barata, L. E. S. 1996. Tropane and pyrrolizidine alkaloids in the ithomiines Placidula euryanassa and Miraleria cymothoe (Lepidoptera: Nymphalidae). Chemoecology 7:61–67.
Green, T. R. and Ryan, C. A. 1972. Wound-induced proteinase inhibitor in plant leaves—possible defense mechanism against insects. Science 175:776–777.
Griffin, W. J. and Lin, G. D. 2000. Chemotaxonomy and geographical distribution of tropane alkaloids. Phytochemistry 53:623–637.
Haukioja, E. 1980. On the role of plant defenses in the fluctuation of herbivore populations. Oikos 35:202–213.
Heil, M. and Baldwin, I. T. 2002. Fitness cost of induced resistance: emerging experimental support for a slippery concept. Trends Plant Sci. 7:61–67.
Hoaglands, D. R. and Arnon, R. I. 1950. The water culture method for growing plant without soil. California Agricultural Experiment Station.
Hsiao, T. H. and Fraenkel, G. 1968. The role of secondary plant substances in the food specificity of the Colorado potato beetle. Ann. Entomol. Soc. Am. 61:485–503.
Lamas, G. 1999. Butterflies of the World. Part 3. Nymphalidae II, Ithomiinae. Goecke & Evers, Keltern.
Lundberg, S., Jaremo, J., and Nilsson, P. 1994. Herbivory, inducible defence and population oscillations: a preliminary theoretical analysis. Oikos 71:537–539.
Karban, R. and Baldwin, I. T. 1997. Induced Responses to Herbivory. University of Chicago Press, Chicago.
Khan, M. B. and Harborne, J. B. 1990. Induced alkaloid defence in Atropa acuminata in response to mechanical and herbivore leaf damage. Chemoecology 1:77–80.
Krug, E. and Proksch, P. 1993. Influence of dietary alkaloids on survival and growth of Spodoptera littoralis. Biochem. Syst. Ecol. 21:749–756.
Macel, M., Bruinsma, M., Dijkstra, S. M., Ooijendijk, T., Niemeyer, H. M., and Klinkhamer, P. G. L. 2005. Differences in effects of pyrrolizidine alkaloids on five generalist insect herbivore species. J. Chem. Ecol. 31:1493–1508.
McKey, D. 1974. Adaptive patterns in alkaloid physiology. Am. Nat. 108:305–320.
McKey, D. 1979. The distribution of secondary compounds within plants, pp. 56–134, in G. A. Rosenthal and D. H. Janzen (eds.). Herbivores: Their Interaction with Secondary Plant Metabolites. Academic Press, New York.
Miller, J. S. and Feeny, P. 1983. Effects of benzylisoquinoline alkaloids on the larvae of polyphagous Lepidoptera. Oecologia 58:332–339.
Mroczek, T., Glowniak, K., and Kowalska, J. 2006. Solid–liquid extraction and cation-exchange solid-phase extraction using a mixed-mode polymeric sorbent of Datura and related alkaloids. J. Chromatogr. A 1107:9–18.
Olmstead, R. G. and Palmer, J. D. 1992. A chloroplast DNA phylogeny of the Solanaceae: subfamilial relationships and character evolution. Ann. Mo. Bot. Gard. 79:346–360.
Reinbothe, S., Mollenhauer, B., and Reinbothe, C. 1994. JlPs and RIPs: the regulation of plant gene expression by jasmonates in response to environmental cues and pathogens. Plant Cell 6:1197–1209.
Rhoades, D. F. 1979. Evolution of plant chemical defense against herbivores, pp. 3–54, in G. A. Rosenthal and D. H. Janzen (eds.). Herbivores: Their Interaction with Secondary Plant Metabolites. Academic Press, New York.
Romero, G. Q. and Izzo, T. J. 2004. Leaf damage induces ant recruitment in the Amazonian ant-plant Hirtella myrmecophila. J. Trop. Biol. 20:675–682.
Shonle, I. 1999. Evolutionary ecology of tropane alkaloids. Ph.D. dissertation. University of Chicago, Chicago, IL.
Shonle, I. and Bergelson, J. 2000. Evolutionary ecology of the tropane alkaloids of Datura stramonium L. (Solanaceae). Evolution 54:778–788.
Underwood, N. C. 1998. The timing of induced resistance and induced susceptibility in the soybean–Mexican bean beetle system. Oecologia 114:376–381.
van Dam, N. M., Meijden E. V. D., and Verpoorte, R. 1993. Induced responses in three alkaloid containing plant species. Oecologia 95:425–430.
van Dam, N. M., de Jong T. J., Iwasa Y., and Kubo, T. 1996. Optimal distribution of defences: are plants smart investors? Funct. Ecol. 10:128–136.
van Dam, N. M., Harvey, J. A., Wäckers, F. L., Bezemer, M., van der Putten, W. H., and Vet, L. E. M. 2003. Interactions between aboveground and belowground induced responses against phytophages. Basic Appl. Ecol. 4:63–77.
van der Meijden, E. 1996. Plant defence, an evolutionary dilemma: contrasting effects of (specialist and generalist) herbivores and natural enemies. Entomol. Exp. Apl. 80:307–310.
Wink, M. 1985. Chemische Verteidigung der Lupinen: zur biologischen Bedeutung der Chinolizidinalkaloide. Plant Syst. Evol. 150:65–81.
Wink, M. 1993. Allelochemical properties or raison d’etre of alkaloids. Alkaloids 43:1–118.
Wink, M. 2003. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 64:3–19.
Young, T. P. 1987. Increased thorn length in Acacia depranolobium: an induced response to browsing. Oecologia 71:436–438.
Zangerl, A. R. and Bazzaz, F. A. 1992. Theory and pattern in plant defense allocation, pp. 363–391, in R. S. Fritz and E. L. Simms (eds.). Plant Resistance to Herbivores and Pathogens. Ecology, Evolution, and Genetics. The University of Chicago Press, Chicago.
Zangerl, A. R. and Rutledge, C. E. 1996. The probability of attack and patterns of constitutive and induced defense: a test of optimal defense theory. Am. Nat. 147:599–608.
Zar, J. H. 1999. Biostatistical analysis. 4th edn. Prentice-Hall, Englewood Cliffs, NJ
Acknowledgments
We thank Flávia Tavares Colpas, Keith S. Brown, Klaas Vrieling, Rodrigo Cogni, Suzana de Fátima Alcântara, and two anonymous reviewers for comments on the manuscript. The research was supported by a Fundação de Amparo à Pesquisa do Estado de São Paulo grant for JRT (98/01065-7). We thank also the Prefeitura Municipal de Jundiaí for permission to work in Serra do Japi. This work is part of the Ph.D. dissertation of MNA at Pós-Graduação em Biologia Vegetal, Instituto de Biologia, Universidade de Campinas.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alves, M.N., Sartoratto, A. & Trigo, J.R. Scopolamine in Brugmansia Suaveolens (Solanaceae): Defense, Allocation, Costs, and Induced Response. J Chem Ecol 33, 297–309 (2007). https://doi.org/10.1007/s10886-006-9214-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-006-9214-9