Abstract
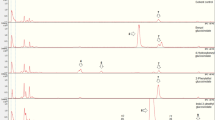
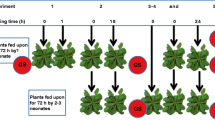
The antiherbivore potential of the glucosinolate–myrosinase defense system found in plants of the order Capparales is heavily influenced by the types of hydrolysis products (e.g. isothiocyanates, nitriles) formed from the parent glucosinolates upon plant damage. However, comparison of the effects of glucosinolate hydrolysis products on insect herbivores has been hampered by the lack of suitable experimental tools for rigorous bioassays, such as intact plants differing only in the types of hydrolysis products they produce, or artificial diets that can accurately simulate glucosinolate hydrolysis. The wide array of molecular resources for Arabidopsis thaliana has facilitated the identification of several genes that play a role in glucosinolate hydrolysis. One of these encodes the epithio-specifier protein (ESP) that promotes the formation of nitriles at the expense of isothiocyanates in certain ecotypes of A. thaliana. We overexpressed the ESP cDNA from the nitrile-producing ecotype Landsberg erecta in the isothiocyanate-producing ecotype Columbia-0 to generate transgenic lines of A. thaliana that differed from wild-type plants in the type of glucosinolate hydrolysis products formed upon tissue damage, whereas parent glucosinolate profile and myrosinase activity levels, as well as plant morphology and growth habit, remained unchanged. Bioassays with the model generalist herbivore Spodoptera littoralis (Lepidoptera: Noctuidae) demonstrated that larvae reared on the nitrile-producing lines on average gained weight faster in the first larval stages than larvae that fed on isothiocyanate-producing control plants. Furthermore, larvae with medial growth rates showed a tendency to pupate earlier on the ESP-overexpressing plant lines. Together with the results of previous studies, these findings suggest that isothiocyanates are more effective defenses against insect herbivores than nitriles, and raise questions about what conditions select for nitrile formation in plants.




Similar content being viewed by others
References
Agrawal, A. A. and Kurashige, N. S. 2003. A role for isothiocyanates in plant resistance against the specialist herbivore Pieris rapae. J. Chem. Ecol. 29:1403–1415.
Bak, S., Olsen, C. E., Halkier, B. A., and Moller, B. L. 2000. Transgenic tobacco and Arabidopsis plants expressing the two multifunctional sorghum cytochrome P450 enzymes, CYP79A1 and CYP71E1, are cyanogenic and accumulate metabolites derived from intermediates in dhurrin biosynthesis. Plant Physiol. 123:1437–1448.
Bartelt, R. J. and Mikolajczak, K. L. 1989. Toxicity of compounds derived from Limnanthes alba seed to fall armyworm (Lepidoptera, Noctuidae) and European corn-borer (Lepidoptera, Pyralidae) Larvae. J. Econ. Entomol. 82:1054–1060.
Blau, P. A., Feeny, P., Contardo, L., and Robson, D. S. 1978. Allylglucosinolate and herbivorous caterpillars—A contrast in toxicity and tolerance. Science 200:1296–1298.
Buskov, S., Serra, B., Rosa, E., Sorensen, H., and Sorensen, J. C. 2002. Effects of intact glucosinolates and products produced from glucosinolates in myrosinase-catalyzed hydrolysis on the potato cyst nematode (Globodera rostochiensis cv. Woll). J. Agric. Food Chem. 50:690–695.
Chew, F. S. 1988. Biological effects of glucosinolates, pp. 155–181, in H. G. Cutler (ed.). Biologically Active Natural Products. American Chemical Society, Washington, DC.
Clough, S. J. and Bent, A. F. 1998. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16:735–743.
Fiebig, H. J. and Arens, M. 1992. Glucosinolate (HPLC-Methode). Fat Sci. Tech. 94:199–203.
Giamoustaris, A. and Mithen, R. 1995. The effect of modifying the glucosinolate content of leaves of oilseed rape (Brassica napus ssp oleifera) on its interaction with specialist and generalist pests. Ann. Appl. Biol. 126:347–363.
Hajdukiewicz, P., Svab, Z., and Maliga, P. 1994. The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol. Biol. 25:989–994.
Hansen, C. H., Wittstock, U., Olsen, C. E., Hick, A. J., Pickett, J. A., and Halkier, B. A. 2001. Cytochrome P450 CYP79F1 from Arabidopsis catalyzes the conversion of dihomomethionine and trihomomethionine to the corresponding aldoximes in the biosynthesis of aliphatic glucosinolates. J. Biol. Chem. 276:11078–11085.
Hasapis, X. and MacLeod, A. J. 1982. Benzylglucosinolate degradation in heat-treated Lepidium sativum seeds and detection of a thiocyanate-forming factor. Phytochemistry 21:1009–1013.
Hogge, L. R., Reed, D. W., Underhill, E. W., and Haughn, G. W. 1988. HPLC separation of glucosinolates from leaves and seeds of Arabidopsis thaliana and their identification using thermospray liquid-chromatography mass-spectrometry. J. Chromatogr. Sci. 26:551–556.
Kliebenstein, D. J., Kroymann, J., Brown, P., Figuth, A., Pedersen, D., Gershenzon, J., and Mitchell-Olds, T. 2001a. Genetic control of natural variation in Arabidopsis glucosinolate accumulation. Plant Physiol. 126:811–825.
Kliebenstein, D. J., Lambrix, V. M., Reichelt, M., Gershenzon, J., and Mitchell-Olds, T. 2001b. Gene duplication in the diversification of secondary metabolism: Tandem 2-oxoglutarate-dependent dioxygenases control glucosinolate biosynthesis in arabidopsis. Plant Cell 13:681–693.
Kroymann, J., Textor, S., Tokuhisa, J. G., Falk, K. L., Bartram, S., Gershenzon, J., and Mitchell-Olds, T. 2001. A gene controlling variation in Arabidopsis glucosinolate composition is part of the methionine chain elongation pathway. Plant Physiol. 127:1077–1088.
Lambrix, V. M., Reichelt, M., Mitchell-Olds, T., Kliebenstein, D. J., and Gershenzon, J. 2001. The Arabidopsis epithiospecifier protein promotes the hydrolysis of glucosinolates to nitriles and influences Trichoplusia ni herbivory. Plant Cell 13:2793–2807.
Li, Q., Eigenbrode, S. D., Stringham, G. R., and Thiagarajah, M. R. 2000. Feeding and growth of Plutella xylostella and Spodoptera eridania on Brassica juncea with varying glucosinolate concentrations and myrosinase activities. J. Chem. Ecol. 26:2401–2419.
Lichtenstein, E. P., Morgan, D. G., and Strong, F. M. 1962. Naturally occurring insecticides—Identification of 2-phenylethylisothiocyanate as an insecticide occurring naturally in edible part of turnips. J. Agric. Food Chem. 10:30–33.
MacLeod, A. J. and Rossiter, J. T. 1985. The occurence and activity of epithiospecifier protein in some Cruciferae seeds. Phytochemistry 24:1895–1898.
Matile, P. 1980. The mustard oil bomb—Compartmentation of the myrosinase system. Biochem. Physiol. Pflanzen. 175:722–731.
Mikkelsen, M. D., Hansen, C. H., Wittstock, U., and Halkier, B. A. 2000. Cytochrome P450 CYP79B2 from Arabidopsis catalyzes the conversion of tryptophan to indole-3-acetaldoxime, a precursor of indole glucosinolates and indole-3-acetic acid. J. Biol. Chem. 275:33712–33717.
Nielsen, J. K., Hansen, M. L., Agerbirk, N., Petersen, B. L., and Halkier, B. A. 2001. Responses of the flea beetles Phyllotreta nemorum and P. cruciferae to metabolically engineered Arabidopsis thaliana with an altered glucosinolate profile. Chemoecology 11:75–83.
Petersen, B. L., Andreasson, E., Bak, S., Agerbirk, N., and Halkier, B. A. 2001. Characterization of transgenic Arabidopsis thaliana with metabolically engineered high levels of p-hydroxybenzylglucosinolate. Planta 212:612–618.
Petroski, R. J. and Kwolek, W. F. 1985. Interaction of a fungal thioglucoside glucohydrolase and cruciferous plant epithiospecifier protein to form 1-cyanoepithioalkanes: Implications of an allosteric mechanism. Phytochemistry 24:213–216.
Rask, L., Andreasson, E., Ekbom, B., Eriksson, S., Pontoppidan, B., and Meijer, J. 2000. Myrosinase: Gene family evolution and herbivore defense in Brassicaceae. Plant Mol. Biol. 42:93–113.
Reichelt, M., Brown, P. D., Schneider, B., Oldham, N. J., Stauber, E., Tokuhisa, J., Kliebenstein, D. J., Mitchell-Olds, T., and Gershenzon, J. 2002. Benzoic acid glucosinolate esters and other glucosinolates from Arabidopsis thaliana. Phytochemistry 59:663–671.
Reintanz, B., Lehnen, M., Reichelt, M., Gershenzon, J., Kowalczyk, M., Sandberg, G., Godde, M., Uhl, R., and Palme, K. 2001. Bus, a bushy Arabidopsis CYP79F1 knockout mutant with abolished synthesis of short-chain aliphatic glucosinolates. Plant Cell 13:351–367.
Schlueter, M. and Gmelin, R. 1972. Abnormale enzymatische Spaltung von 4-Methylthiobutylglucosinolat in Frischpflanzen von Eruca sativa. Phytochemistry 11:3427–3431.
Seo, S. T. and Tang, C. S. 1982. Hawaiian fruit-flies (Diptera, Tephritidae)—Toxicity of benzyl isothiocyanate against eggs or 1st instars of three species. J. Econ. Entomol. 75:1132–1135.
Spencer, G. F. and Daxenbichler, M. E. 1980. Gas chromatography-mass spectrometry of nitriles, isothiocyanates and oxazolidinethiones derived from cruciferous glucosinolates. J. Sci. Food Agric. 31:359–367.
Stoewsand, G. S. 1995. Bioactive organosulfur phytochemicals in Brassica oleracea vegetables—A review. Food Chem. Toxicology 33:537–43.
Stowe, K. A. 1998. Realized defense of artificially selected lines of Brassica rapa: Effects of quantitative genetic variation in foliar glucosinolate concentration. Environ. Entomol. 27:1166–1174.
Tattersall, D. B., Bak, S., Jones, P. R., Olsen, C. E., Nielsen, J. K., Hansen, M. L., Hoj, P. B., and Moller, B. L. 2001. Resistance to an herbivore through engineered cyanogenic glucoside synthesis. Science 293:1826–1828.
Tierens, K., Thomma, B. P. H., Brouwer, M., Schmidt, J., Kistner, K., Porzel, A., Mauch-Mani, B., Cammue, B. P. A., and Broekaert, W. F. 2001. Study of the role of antimicrobial glucosinolate-derived isothiocyanates in resistance of Arabidopsis to microbial pathogens. Plant Physiol. 125:1688–1699.
Tookey, H. L. 1973. Crambe thioglucoside glucohydrolase (EC3.2.3.1)—Separation of a protein required for epithiobutane formation. Can. J. Biochem. 51:1654–1660.
Töpfer, R., Matzeit, V., Gronenborn, B., Schell, J., and Steinbiss, H. H. 1987. A set of plant expression vectors for transcriptional and translational fusions. Nuc. Acids Res. 15:5890–5890.
Wathelet, J. P., Iori, R., Leoni, O., Rollin, P., Quinsac, A., and Palmieri, S. 2004. Guidelines for glucosinolate analysis in green tissues used for biofumigation. Agroindustria 3:257–266.
Wittstock, U. and Halkier, B. A. 2000. Cytochrome P450 CYP79A2 from Arabidopsis thaliana L. catalyzes the conversion of l-phenylalanine to phenylacetaldoxime in the biosynthesis of benzylglucosinolate. J. Biol. Chem. 275:14659–14666.
Wittstock, U., Kliebenstein, D., Lambrix, V. M., Reichelt, M., and Gershenzon, J. 2003. Glucosinolate hydrolysis and its impact on generalist and specialist insect herbivores, pp. 101–126, in J. T. Romeo (ed.). Recent Advances in Phytochemistry—Integrative Phytochemistry: From Ethnobotany to Molecular Ecology. Elsevier, Amsterdam.
Zabala, M. D., Grant, M., Bones, A. M., Bennett, R., Lim, Y. S., Kissen, R., and Rossiter, J. T. 2005. Characterisation of recombinant epithiospecifier protein and its over-expression in Arabidopsis thaliana. Phytochemistry 66:859–867.
Zhang, Y. S., Wade, K. L., Prestera, T., and Talalay, P. 1996. Quantitative determination of isothiocyanates, dithiocarbamates, carbon disulfide, and related thiocarbonyl compounds by cyclocondensation with 1,2-benzenedithiol. Anal. Biochem. 239:160–167.
Acknowledgments
We thank Andrea Bergner for technical assistance, Michael Reichelt for providing intact glucosinolates, and the Max Planck Society for financial support. Two anonymous reviewers are thanked for their valuable comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burow, M., Müller, R., Gershenzon, J. et al. Altered Glucosinolate Hydrolysis in Genetically Engineered Arabidopsis thaliana and its Influence on the Larval Development of Spodoptera littoralis . J Chem Ecol 32, 2333–2349 (2006). https://doi.org/10.1007/s10886-006-9149-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-006-9149-1