Abstract
Oxygenation through High Flow Delivery Systems (HFO) is described as capable of delivering accurate FiO2. Meanwhile, peak inspiratory flow \(\dot{V}_{{\text{I}}}\) ) of patients with acute hypoxemic respiratory failure can reach up to 120 L/min, largely exceeding HFO flow. Currently, very few data on the reliability of HFO devices at these high \(\dot{V}_{{\text{I}}}\) are available. We sought to evaluate factors affecting oxygenation while using HFO systems at high \(\dot{V}_{{\text{I}}}\) in a bench study. Spontaneous breathing was generated with a mechanical test lung connected to a mechanical ventilator Servo-i®, set to volume control mode. Gas flow from a HFO device was delivered to the test lung. The influence on effective inspired oxygen fraction of three parameters (FiO2 0.6, 0.8, and 1, \(\dot{V}_{{\text{I}}}\) from 28 to 98.1 L/min, and HFO Gas Flows from 40 to 60 L/min) were analyzed and are reported. The present bench study demonstrates that during HFO treatment, measured FiO2 in the lung does not equal set FiO2 on the device. The substance of this variation (ΔFiO2) is tightly correlated to \(\dot{V}_{{\text{I}}}\) (Pearson’s coefficient of 0.94, p-value < 0.001). Additionally, set FiO2 and Flow at HFO device appear to significatively affect ΔFiO2 as well (p-values < 0.001, adjusted to \(\dot{V}_{{\text{I}}}\) ). The result of multivariate linear regression indicates predictors (\(\dot{V}_{{\text{I}}}\) , Flow and set FiO2) to explain 92% of the variance of delta FiO2 through K-Fold Cross Validation. Moreover, adjunction of a dead space in the breathing circuit significantly decreased ΔFiO2 (p < 0.01). The present bench study did expose a weakness of HFO devices in reliability of delivering accurate FIO2 at high \(\dot{V}_{{\text{I}}}\) as well as, to a lesser extent, at \(\dot{V}_{{\text{I}}}\) below equivalent set HFO Flows. Moreover, set HFO flow and set FIO2 did influence the variability of effective inspired oxygen fraction. The adjunction of a dead space in the experimental set-up significantly amended this variability and should thus be further studied in order to improve success rate of HFO therapy.
Similar content being viewed by others
1 Introduction
Oxygenation through high flow delivery (HFO) systems has been gaining increasing interest for the last 10 years among intensivists and emergency care physicians, especially in the context of advanced acute respiratory failure resistant to low flow oxygen therapy [1, 2]. The HFO devices are described as capable of delivering reliable, reproducible and accurate FiO2 from 0.21 to 1.0 with flows between 20 and 60 L/min [3, 4]. Hypoxemic critically ill patients do benefit the most of high flow oxygen devices and present with high Minute Ventilation (\(\dot{V}_{{\text{E}}}\) ) and consequently high peak inspiratory flows (\(\dot{V}_{{\text{I}}}\) ) [5]. This is especially the case of critically ill patients due to the Coronavirus 2019 disease (SARS-CoV-2). Indeed, despite severe pulmonary damage, the latter patients can maintain quite normal elastic-resistive thoraco-pulmonary characteristics during the first week of respiratory failure [6] thus being often capable of maintaining high \(\dot{V}_{{\text{E}}}\) and \(\dot{V}_{{\text{I}}}\) during a prolonged time [7]. In some cases, the peak inspiratory flow of patients with acute hypoxemic respiratory failure can even reach up to 120 L/min [8], largely exceeding HFO flow. At these high \(\dot{V}_{{\text{I}}}\) , the assumption is made that atmospheric room air might enter the breathing circuit and dilute the set FiO2 from HFO [3] reducing consequently the effectively delivered FIO2.
We firmly believe that a thorough understanding of FiO2 stability during HFO therapy is paramount as it is increasingly used in acute respiratory failure severity scores [9] [10] as well as scores predicting the need for intubation [11]. Since HFO is used in very sick hypoxemic patients, with high Respiratory frequency (Rf) and high Tidal Volumes (Vt) due to high oxygen demands and thus approximate \(\dot{V}_{{\text{I}}}\) up to 100 L/min [12], such as critically ill SARS-CoV-2 patients, we sought to evaluate the reliability of HFO systems at these higher \(\dot{V}_{{\text{I}}}\) .
Therefore, the aim of the study was to analyze the effect of high \(\dot{V}_{{\text{E}}}\) and \(\dot{V}_{{\text{I}}}\) on effective FIO2 during HFO treatment in an experimental adult bench model.
Additionally, since previous studies have found that the addition of a surgical mask or a Double Trunk Mask (DTM) (dead space) above HFO nasal cannulas may increase the arterial pressure in oxygen in some hypoxemic patients despite any modification in HFO settings [13, 14], the secondary aim of the study was to evaluate the impact of the addition of a dead space on effective inspired FiO2.
2 Material and methods
2.1 Experimental adult model
The HFO was generated by an Airvo2 ™ device (Fisher & Paykel Health Care, Auckland, New Zealand) through a T piece connector (INTERSURGICAL T-Piece Connector iso 22-DS00586). The T piece was connected to a mechanical test lung through a single ventilator tubing (iso 22). The first lung was driven by a mechanical ventilator (Servoi™ Maquet, Getinge group, Getingue, Sweden) (Labelled as Drive Mechanical Ventilator) to simulate a spontaneous breathing pattern.
2.2 High flow oxygenation device (HFO)
Gas flow from Airvo2 ™ was set at 40, 50 and 60 L/min and confirmed by a calibrated flowmeter (SP-304 flow sensor-Iworx®, United States). FiO2 was set at 0.6, 0.8 and 1 and confirmed by a calibrated oxygen analyzer (two point calibration, GA-200 Side stream, Gas Analyzer Iworx®, United States) which was connected to a data-acquisition system IX-214 hardware Oxygen analyzer GA-200, first calibrated with room air (21%) and secondly at 100% with certified O2 gas coming from an oxygen gas cylinder. To evaluate the dynamic variability of effective inspired oxygen fraction into this bench model during respiratory cycle, the measures of FiO2 were performed at two different locations:
-
(1)
Directly into the artificial lung through the O2 port of the second lung (Fig. 1)
-
(2)
Near the T piece (Fig. 1).
When an experimental setting was changed, it was left stabilizing for 3 min prior to measuring. The FIO2 was measured for 1 min and data of the final 3 breaths were extracted. The mean of these three measures is reported.
2.3 Spontaneous breathing
Spontaneous breathing was generated in Ambient Temperature and Pressure Saturated (ATPS) conditions with a mechanical test lung (Dual Test Lung-Michigan Instruments, Inc. Grand Rapids Model 5600i) including two independent artificial lungs. The two lung compartments were synchronized with a lung coupling clip, which allowed the first lung to drive the second lung in order to achieve spontaneous breathing simulation. Resistive and Elastic characteristics of Dual Test Lung were: 5 cmH2O/L/sec and 0.06 L/cmH2O.
The first lung was driven by a mechanical ventilator Servo-i™ (Servo i™ Maquet, Getinge group, Getinge, Sweden) set to volume control mode (descending ramp flow waveform without auto-flow, time pause and inspiratory rise time at 0%, peep of 0 cmH2O, the trigger was set at –10 cmH20 in order to avoid self-triggering).
Respiratory frequency (Rf) was set to 10, 15, 20, 25, 30 and 35 breaths/min with an inspiratory time (Ti) to expiratory time (Te) ratio of 0.5. Tidal Volume was set at 0.7 L giving \(\dot{V}_{{\text{E}}}\) equal to 7, 10.5, 14, 17.5, 21 and 24.5 L/min, either \(\dot{V}_{{\text{I}}}\) of 28, 42, 56.1, 70.1, 84.1, 98.1 L/min respectively.
The experiment was duplicated with the adjunction of a supplementary dead space of 0.230L (corrugated ISO 22 tube of 30 cm of length) connected on the T-piece, corresponding to the “With Trunk” set-up (Fig. 1).
2.4 Statistical analysis
\(\dot{V}_{{\text{E}}}\) was defined as the product of Vt and Rf. Difference in measured FiO2 (mFiO2) and set FiO2 (sFiO2) was defined as ΔFiO2. Continuous variables are presented as mean (± SD). The assessment of the relationship between \(\dot{V}_{{\text{I}}}\) and ΔFiO2 was computed through Pearson’s correlation analysis in function of the different variable groups (set HFO Flow and sFiO2). To define which variables were the most determinant in predicting effective inspired oxygen concentration and ΔFiO2, forward stepwise multiple linear regression was computed. Variables with a p-value < 0.01 were entered into the model. Multiple linear regression modelling was computed to evaluate the effects of selected variables (\(\dot{V}_{{\text{E}}}\) , HFO Flow, and sFiO2) on ΔFiO2 and effective inspired oxygen fraction. The model was then internally validated with a 10-Fold Cross Validation Procedure.
Differences between groups (with and without Trunk, sFiO2 and HFO Flow) were compared through ANCOVA adjusted for \(\dot{V}_{{\text{I}}}\) . Normality was assessed using the Shapiro–Wilk test and linearity between the dependent variable (ΔFiO2) and the covariate (\(\dot{V}_{{\text{I}}}\) ) was assessed by correlation plots with Pearson’s correlation coefficient per studied factor (Trunk, set HFO Flow and sFiO2).
Throughout analysis, a p-value below 0.01 was considered as statistically significant.
Statistical analysis was performed using R: A Language and Environment for Statistical Computing, R Core Team, Vienna, Austria, 2020, https://www.r-project.org/.
(Used packages emmeans, ggpubr, rstatix, lattice, car, caret).
3 Results
The influence of the different variables on ΔFiO2 is demonstrated in Fig. 2; Tables 1 and 2. The experimental set up demonstrated high variation in measured FiO2 (mFiO2) through the respiratory cycle in function of the location of the measurement. At the T-piece, measured FiO2 oscillated from ± 10% whereas, at the lung sampling point, this oscillation was around 2–4%. Therefore, further FiO2 measurements were conducted intra-pulmonary.
The difference (ΔFiO2) between measured FiO2 and sFiO2, is influenced by \(\dot{V}_{{\text{I}}}\) , set Flow and FiO2 at HFO (Fig. 4; Table 2).
\(\dot{V}_{{\text{I}}}\) exerted a positive linear relation with ΔFiO2 throughout the different variable groups (set HFO Flows and sFiO2) with a minimal Pearson’s coefficient of 0.94 (p-value < 0.001). The higher \(\dot{V}_{{\text{I}}}\) , the more ΔFiO2 increases. This trend is accentuated with the increase of sFiO2 and the decrease of HFO Flow (Fig. 3), reaching a maximum ΔFiO2 of 44% at \(\dot{V}_{{\text{I}}}\) of ± 98 L/min, sFiO2 at 1 with a HFO flow of ± 40 L/min. For each curve without trunk reported in Fig. 3, there seems to be a threshold under which ΔFiO2 variations are less important.
ΔFiO2 in function of Peak Inspiratory Flow grouped by the different experimental variables (sFiO2 and HFO Flow). The blue curve represent ΔFiO2 variations without adjunction of a dead space (Without Trunk) as the red curve does represent ΔFiO2 with the adjunction of a dead space (With Trunk). ΔFiO2 rises when Flows are lower and set FiO2 is high. Dashed line represents Peak Inspiratory Flow equal to HFO Flow
This threshold matches the point at which \(\dot{V}_{{\text{I}}}\) equalize HFO Flow (intersection of dashed line and without trunk curve in Fig. 3).
Stepwise selection model (Table 1) showed \(\dot{V}_{{\text{I}}}\) to be the most influencing variable of ΔFiO2. The model did improve significantly with the adjunction of HFO Flow and set FiO2: R2 increases, and a Root-Mean-Square Error (RMSE) decreases at each step.
The model, with ΔFiO2 as dependent variable, based on the stepwise selection model (Table 2) yielded a R2 of 0.849 and a RMSE 3.83. This could be improved with a 10-Fold Cross Validation procedure to a R2 of 0.92 and a RMSE of 3.76. All three variables (\(\dot{V}_{{\text{E}}}\) , sFiO2, and HFO Flow) are highly significant in predicting ΔFiO2 (p < 0.001).
Effects on ΔFiO2 of HFO Flow and sFiO2, adjusted to \(\dot{V}_{{\text{I}}}\) (estimated marginal means), is shown in Fig. 4. Set FiO2 significantly modified ΔFiO2 (ANCOVA adjusted for \(\dot{V}_{{\text{I}}}\) , F = 35.45 p < 0.001). On the other hand, HFO Flow also did significantly change ΔFiO2 (ANCOVA adjusted for \(\dot{V}\) E, F = 48.14, p < 0.001).
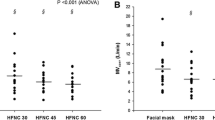
Estimated Marginal Means of ΔFiO2, after adjustment of \(\dot{V}_{{\text{I}}}\) , in function of sFiO2, at HFO Flow 40, 50 and 60 L/min. ΔFiO2 = Difference between Set FiO2 (sFiO2) and measured FiO2 (mFiO2), HFO = High Flow Oxygenation. Differences between flows are statistically significant (cfr ANCOVA result). At different levels of Set FiO2, differences in ΔFiO2 is most important with HFO flow at 40L/min
When a trunk was added to the T-piece, ΔFiO2 did significantly decrease at each level of sFiO2 (Tukey’s adjusted p-value < 0.01) (Fig. 5A). ΔFiO2 did also decrease at each different set Flow at high oxygenation device with adjunction of a trunk (Tukey’s adjusted p-value < 0.01) (Fig. 5B).
4 Discussion
The present bench study demonstrates that during HFO treatment, measured FiO2 in the lung does not always equal set FiO2 on the device. The substance of this variation seems to be directly correlated to \(\dot{V}_{{\text{I}}}\) value. Indeed, as \(\dot{V}_{{\text{I}}}\) increases, ΔFiO2 amplifies. Additionally, sFiO2 and Flow at HFO device appear to significatively affect this difference. The result of the linear regression indicated predictors (\(\dot{V}_{{\text{I}}}\) , Flow and set FiO2) to explain 92% of the variance of ΔFiO2 through K-Fold Cross Validation. Interestingly, adjunction of a dead space in the breathing circuit did significantly decrease ΔFiO2.
At first, this study shows important oscillations in FiO2 measures through the respiratory cycle in function of the location of the measurements in the experimental set-up (Fig. 1). To simulate physiological conditions, i.e. open mouth or inevitable leaks around the nasal cannula, a T-piece was mounted in the experimental set up. FiO2 measurements at the T-piece present oscillation of up to 10% in FiO2 through the respiratory cycle in comparison with oscillations of up to 4% of “intra-pulmonary” measurements (Fig. 1). This first finding demonstrates presence of air blending in this part of the circuit, that stabilizes when arriving in the lungs. Analysis in the present study was therefore carried out on stabilized intra-pulmonary FiO2 measurements, as recommended by the manufacturer of the Dual Test Lung.
Through the analysis of these measurements, important differences were found between sFiO2 and mFiO2 (sFiO2—mFiO2 = ΔFiO2) at different \(\dot{V}_{{\text{I}}}\) , Flow and set FiO2. \(\dot{V}_{{\text{I}}}\) was the most significant variable influencing ΔFiO2 and exerted a positive linear relation with the latter. This finding accentuates the purpose of our study which assessed the effects of high \(\dot{V}_{{\text{E}}}\) and \(\dot{V}_{{\text{I}}}\) while other studies did limit this variable to physiologic “resting” values [3].
Meanwhile, there seems to be a threshold of \(\dot{V}_{{\text{I}}}\) under which ΔFiO2 does diverge less. It was indeed previously hypothesized that once \(\dot{V}_{{\text{I}}}\) do exceed HFO Flow of the High Flow Oxygenation Device, air blending between high oxygenated air from HFO and room air from the mouth or leaks do occur and could thus decreases actual delivered FiO2 [3]. When \(\dot{V}_{{\text{I}}}\) does exceed HFO Flow, depression happen inside the circuit, which consequently causes a suction effect inside the upper airways. Entrained room-air arriving from the mouth or leaks around the nasal canula (the T piece in our experimental set up), blend with oxygenated air from HFO, thus decreasing FiO2 of inspired HFO flow.
From our data we can indeed extrapolate a breaking point corresponding to the point where \(\dot{V}_{{\text{I}}}\) and HFO Flow equalizes. This point can be found at the intersection of the dashed line (\(\dot{V}_{{\text{I}}}\) = HFO Flow) and the curve “Without Trunk” in Fig. 3. If above this point ΔFiO2 looks clear and was expected, it seems however that below this point, air blending does also occur.
To explain the latter, we make the assumption of a possible Bernoulli effect in the upper airways during inspiration when \(\dot{V}_{{\text{I}}}\) does not exceed HFO Flow. Laminar flux creates depression in the circuit and thus an air suction effect coming from the T-piece (anatomically comparable with the mouth or leaks around nasal cannula). Air blending will occur and generate a ΔFiO2 which value will vary on sFiO2 and HFO Flow (Fig. 3.). Indeed, ΔFiO2 will increase when sFiO2 is set at higher values, and at lower HFO Flows.
The rational principle behind these different findings lays on the mathematical air-mixing equation of an air circuit [15]. With the formula of this principle, we simulated and depicted in Fig. 6 the relationship of FiO2 calculated through different sFiO2 at different Air Flows. Air Flow in this simulation illustrates the flow arising from the suction effect during either assumed Bernoulli effect either \(\dot{V}_{{\text{I}}}\) exceeding HFO Flow. As the mean difference in \(\dot{V}_{{\text{I}}}\) and set HFO Flow was of 13L/min in our experiment, we choose to represent Air Flows of 5, 12.5 and 20 L/min. In this simulation, we see that the lower sFiO2, the less it will be affected by room air blending, and thus, the difference in sFiO2 and calculated FiO2 will remain small. In the same way, the higher the Air Flow, the more air blending occurs, and thus, increasing difference between sFiO2 and calculated FiO2. This is in accordance with our findings that higher sFiO2 and lower HFO flows tend to augment ΔFiO2.
Clinical applications of our findings are important. Indeed, how sicker the patients, the more important \(\dot{V}_{{\text{I}}}\) and the need of high FiO2, the less reliable FiO2 delivery from HFO become. The only parameter limiting differences in sFiO2 and actual FiO2 in patients with high \(\dot{V}_{{\text{I}}}\) seem to be the flow rate at HFO device. We could therefore suggest augmenting flow in these patients before trying to augment FiO2 to diminish room-air suction effect of patients with major peak inspiratory flow. The use of devices with the ability to deliver higher Flows could also be determinant in assuring reliable FiO2 to patients with high \(\dot{V}_{{\text{I}}}\) .
Another objective of our study was to analyze the effect of the adjunction of a dead space in the circuit. The addition of a tube ISO 22 of 30 cm of length (0.230 L) on the T-piece did significantly decrease ΔFiO2. This could be explained by the fact that during expiration, high oxygenated air from HFO will be contained in the dead spaces (mouth, surgical mask or Double Trunk Mask). According to the air-mixing theorem, during inspiration, with Inspiratory Flow above HFO Flow, instead of room air at a fraction of 0.21 of oxygen, air at higher oxygen fractions will blend with high oxygenated air from HFO, thus limiting ΔFiO2. Hence, adjunction of a dead space (Double Trunk Mask or equivalent) could also be a promising premise to enhance HFO therapy as it was already clinically demonstrated [13, 14] and should be further studied.
The present study has limitations. In clinical practice it is difficult to appreciate actual \(\dot{V}_{{\text{E}}}\) and estimate the Ti on total respiratory cycle time (Ti + Te) ratio which determine mean inspiratory flow and could help estimating peak inspiratory flow. Furthermore, as it is a bench study, the clinical consequences can only be hypothesized and warrant further in vivo research.
Our model does not exactly reproduce three-dimensional anatomy of the upper airways. Nevertheless, in physiological situation, we expect less ideal gas flows than our experimental design. Hence, we expect more turbulent flows in patient’s upper airways, which only can augment our estimate ΔFiO2 through amplifying air blending.
5 Conclusion
The present bench study did expose a weakness of HFO devices in reliability of delivering accurate effective inspired FIO2 at high \(\dot{V}_{{\text{I}}}\) as well as, to a lesser extent, at \(\dot{V}_{{\text{I}}}\) below equivalent HFO Flows. Moreover, set HFO flow and sFIO2 did influence variability of effective inspired oxygen fraction. Until further in vivo research of stability and reliability of effective inspired oxygen fraction during HFO treatment, we advocate to use sFIO2 with caution in severity scores and scores predicting the need for intubation.
Nevertheless, the adjunction of a dead space in the experimental set-up significantly amended variability between sFIO2 and effective inspired FiO2 and could thus be a promising start point to improve success rate of HFO therapy.
Data availability
The dataset used and analyzed during this study are available from the corresponding author on reasonable request.
Abbreviations
- HFO:
-
Oxygenation through High Flow System
- \(\dot{V}_{{\text{I}}}\) :
-
Peak Inspiratory Flow
- \(\dot{V}_{{\text{E}}}\) :
-
Minute Ventilation
- FiO2 :
-
Inspired oxygen fraction
- sFiO2 :
-
Set inspired oxygen fraction at HFO device
- mFiO2 :
-
Measured inspired oxygen fraction in the test lung (or effective inspired oxygen fraction)
- ΔFiO2 :
-
Difference between set inspired oxygen fraction and measured inspired oxygen fraction
References
Ward JJ. High-flow oxygen administration by nasal cannula for adult and perinatal patients. Respir Care. 2013;58(1):98–120.
Nishimura M. High-flow nasal cannula oxygen therapy in adults. J Intensive Care. 2015;3(1):1–8.
Chikata Y, Onodera M, Oto J, Nishimura M. FIO2 in an adult model simulating high-flow nasal cannula therapy. Respir Care. 2017;62(2):193–8.
Nishimura M. High-flow nasal cannula oxygen therapy in adults: physiological benefits, indication, clinical benefits, and adverse effects. Respir Care. 2016. https://doi.org/10.4187/respcare.04577.
Katz JA, Marks JD. Inspiratory work with and without continuous positive airway pressure in patients with acute respiratory failure. Anesthesiology. 1985;63(6):598–607.
Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. COVID-19 does not lead to a “Typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201(10):1299–300.
Mæhlen JO, Mikalsen R, Heimdal HJ, Rehn M, Hagemo JS, Ottestad W. Pre-hospital critical care management of severe hypoxemia in victims of Covid-19: a case series. Scand J Trauma Resusc Emerg Med. 2021;29(1):16.
L’Her E, Deye N, Lellouche F, Taille S, Demoule A, Fraticelli A, et al. Physiologic effects of noninvasive ventilation during acute lung injury. Am J Respir Crit Care Med. 2005;172(9):1112–8.
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–10.
Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–33.
Roca O, Caralt B, Messika J, Samper M, Sztrymf B, Hernández G, et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy. Am J Respir Crit Care Med. 2019;199(11):1368–76.
Goldberg S, Ollila HM, Lin L, Sharifi H, Rico T, Andlauer O, et al. Analysis of hypoxic and hypercapnic ventilatory response in healthy volunteers. PLoS One. 2017;12(1):e0168930.
Duprez F, Bruyneel A, Machayekhi S, Droguet M, Bouckaert Y, Brimioulle S, et al. The double-trunk mask improves oxygenation during high-flow nasal cannula therapy for acute hypoxemic respiratory failure. Respir Care. 2019;64(8):908–14.
Montiel V, Robert A, Robert A, Nabaoui A, Marie T, Mestre NM, et al. Surgical mask on top of high-flow nasal cannula improves oxygenation in critically ill COVID-19 patients with hypoxemic respiratory failure. Ann Intensive Care. 2020. https://doi.org/10.1186/s13613-020-00744-x.
Guillaume DW, DeVries D. Ambient pressure air/oxygen blender. J Biomed Eng. 1992;14(2):153–6.
Funding
The study did not receive any funds of any kind.
Author information
Authors and Affiliations
Contributions
CT, FD, WP designed the study, acquired data and wrote the manuscript. Profs XW, GR, AS and GC wrote, read and approved the final manuscript. Drs CT, SM and VB have contributed to bench study conception and statistical calculation.
Corresponding authors
Ethics declarations
Conflict of interest
None of the Authors have any potential conflict of interest related to the manuscript.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Duprez, F., de Terwangne, C., Bellemans, V. et al. High-flow nasal cannula therapy, factors affecting effective inspired oxygen fraction: an experimental adult bench model. J Clin Monit Comput 36, 1441–1448 (2022). https://doi.org/10.1007/s10877-021-00784-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-021-00784-z