Abstract
It remains unclear whether reduced myocardial contractility, venous dilation with decreased venous return, or arterial dilation with reduced systemic vascular resistance contribute most to hypotension after induction of general anesthesia. We sought to assess the relative contribution of various hemodynamic mechanisms to hypotension after induction of general anesthesia with sufentanil, propofol, and rocuronium. In this prospective observational study, we continuously recorded hemodynamic variables during anesthetic induction using a finger-cuff method in 92 non-cardiac surgery patients. After sufentanil administration, there was no clinically important change in arterial pressure, but heart rate increased from baseline by 11 (99.89% confidence interval: 7 to 16) bpm (P < 0.001). After administration of propofol, mean arterial pressure decreased by 23 (17 to 28) mmHg and systemic vascular resistance index decreased by 565 (419 to 712) dyn*s*cm−5*m2 (P values < 0.001). Mean arterial pressure was < 65 mmHg in 27 patients (29%). After propofol administration, heart rate returned to baseline, and stroke volume index and cardiac index remained stable. After tracheal intubation, there were no clinically important differences compared to baseline in heart rate, stroke volume index, and cardiac index, but arterial pressure and systemic vascular resistance index remained markedly decreased. Anesthetic induction with sufentanil, propofol, and rocuronium reduced arterial pressure and systemic vascular resistance index. Heart rate, stroke volume index, and cardiac index remained stable. Post-induction hypotension therefore appears to result from arterial dilation with reduced systemic vascular resistance rather than venous dilation or reduced myocardial contractility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Intraoperative hypotension is associated with myocardial injury, acute kidney injury, and death [1,2,3,4,5,6,7]. The harm threshold appears to be a mean arterial pressure of about 65 mmHg, with risk progressively increasing at lower pressures and longer durations [8]. About a third of all intraoperative hypotension occurs between anesthetic induction and surgical incision [9, 10]. Since surgery has yet to start when this post-induction hypotension occurs, it is largely determined by patients’ baseline risk and anesthetic management [10, 11]—with the latter being modifiable.
Anesthesia is often induced with a combination of sufentanil, propofol, and rocuronium. The neuromuscular blocking agent rocuronium probably has little effect on arterial pressure besides hemodynamic effects related to paralysis itself [12]. However, opioids promote post-induction hypotension [11, 13], as does propofol [14,15,16,17]. It remains unclear, though, whether post-induction hypotension is primarily due to reduced myocardial contractility, venous dilation with decreased venous return, or arterial dilation with reduced systemic vascular resistance [18,19,20]. The relative contribution of different potential pathophysiologic mechanisms to hypotension after anesthetic induction thus remain unclear.
A better understanding of pathophysiologic mechanisms contributing to post-induction hypotension may guide management and reduce hypotension. We therefore sought to assess the relative contribution of various hemodynamic mechanisms to hypotension after induction of general anesthesia with sufentanil, propofol, and rocuronium in adults having non-cardiac surgery.
2 Methods
2.1 Study design
This was a prospective observational study performed in the Department of Anesthesiology, Center of Anesthesiology and Intensive Care Medicine, University Medical Center Hamburg-Eppendorf, Hamburg, Germany between April and August 2018. The study was approved by the Ethics Committee of the Medical Association of Hamburg on January 9, 2018. All patients provided written informed consent. This observational study adheres to the STROBE guidelines.
2.2 Inclusion and exclusion criteria
We included adults with American Society of Anesthesiologists (ASA) physical status class I-III scheduled for elective gynecologic, urologic, otolaryngologic, or oral and maxillofacial surgery with general anesthesia and tracheal intubation. Patients were excluded if they had heart failure (New York Heart Association Functional Classification class II or higher), atrial fibrillation or other high-grade cardiac arrhythmias, peripheral artery occlusive disease (Fontaine stage II or higher), took beta blockers, had edema of the hands or fingers, had a history or suspicion of difficult airway, or an indication for rapid sequence induction. Patients were also excluded when regional anesthesia was performed before induction of anesthesia.
2.3 Study protocol and measurements
Patients were not premedicated. Preoxygenation was performed with a sealed face mask at a positive end-expiratory pressure of 5 mbar. Anesthesia was induced with sufentanil (0.2–0.5 µg*kg−1), propofol (1.5–2.5 mg*kg−1), and rocuronium (0.5–0.9 mg*kg−1). Patients' tracheas were intubated and mechanical ventilation was initiated with a tidal volume of 6–8 mL*kg−1 at a positive end-expiratory pressure of 5 mbar. After induction, general anesthesia was maintained with either propofol or inhaled sevoflurane.
In addition to routine anesthetic monitoring, we continuously measured hemodynamic variables using a non-invasive finger-cuff method (CNAP; CNSystems Medizintechnik GmbH, Graz, Austria). The CNAP system was calibrated to brachial arterial pressure obtained from the system's upper-arm cuff. The CNAP system provides continuous arterial pressure values and waveforms. Using pulse wave analysis, the CNAP system also estimates advanced hemodynamic variables including cardiac output and systemic vascular resistance. The CNAP system was validated in several clinical studies showing that it reliably estimates arterial pressure and cardiac output [21,22,23,24,25].
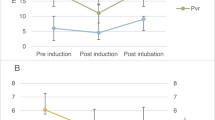
We recorded arterial pressure, heart rate, cardiac index, stroke volume index, and systemic vascular resistance index at the following time points (Fig. 1): before induction of general anesthesia, during preoxygenation, 45 s after administration of sufentanil, 45 s after administration of propofol, 90 s after administration of rocuronium, 60 s after tracheal intubation, and 180 s after tracheal intubation.
Measurement time points. We recorded hemodynamic variables before induction of general anesthesia, during preoxygenation, 45 s after administration of sufentanil, 45 s after administration of propofol, 90 s after administration of rocuronium, 60 s after tracheal intubation, and 180 s after tracheal intubation
2.4 Statistical analysis
Data are presented as mean ± standard deviation (SD) for continuous variables and n (%) for categorical variables. Linear mixed effects models were used to estimate change from baseline (i.e., before induction of anesthesia) in various hemodynamic variables to 6 time points during induction using an autoregressive (AR (1)) covariance structure. The overall significance level was 0.05; Bonferroni correction was used to control the type I error for 7 outcomes and 6 comparisons within each outcome, and the significant level for each comparison was 0.0011 (i.e., alpha = 0.05/7/6 = 0.0011).
3 Results
We enrolled 125 patients but excluded 28 who were given norepinephrine (14 patients) or additional doses of propofol (14 patients) during the study period. We also excluded 5 patients because of technical problems during data recording. We thus included 92 patients in the final analysis.
Participating patients were young, with a mean ± SD age of 36 ± 13 years and relatively healthy with 91% having ASA physical status class I or II (Table 1). General anesthesia was induced with 35 ± 6 µg of sufentanil, 187 ± 39 mg of propofol, and 37 ± 8 mg of rocuronium.
Hemodynamic variables at specified time points are shown in Fig. 2 and Supplemental Table S1. Patients were normotensive at baseline with mean arterial pressure being 96 ± 13 mmHg. At baseline, heart rate was 72 ± 13 bpm, cardiac index was 3.2 ± 0.6 L*min−1*m−2, stroke volume index was 45 ± 6 mL*m−2, and systemic vascular resistance index was 2309 ± 544 dyn*s*cm−5*m2.
Hemodynamic variables during the induction of general anesthesia. Boxplots showing mean (triangle) and median (horizontal bar) with 25th–75th percentile (box) of hemodynamic variables during the induction of general anesthesia. Whiskers extend to the most extreme observations within 1.5 times the interquartile range of the first and third quartiles, respectively. Circles represent outliers. MAP mean arterial pressure, SAP systolic arterial pressure, DAP diastolic arterial pressure, SVRI systemic vascular resistance index, HR heart rate, SVI stroke volume index, CI cardiac index
After sufentanil administration, heart rate increased from baseline by 11 (99.89% confidence interval: 7 to 16) bpm (P < 0.001). As there was no clinically important change in stroke volume index after sufentanil administration the increase in heart rate resulted in a slight increase in cardiac index of 0.5 (0.3 to 0.7) L*min−1m−2. There was no clinically important change in arterial pressure after sufentanil administration.
After administration of propofol, mean arterial pressure decreased by 23 (17 to 28) mmHg and systemic vascular resistance index decreased by 565 (419 to 712) dyn*s*cm-5*m2 (P values < 0.001). After propofol administration, mean arterial pressure was < 65 mmHg in 27 patients (29%). Heart rate returned to baseline after administration of propofol, and stroke volume index and cardiac index remained stable compared to baseline.
After administration of rocuronium, mean arterial pressure, systemic vascular resistance index, and heart rate all were below baseline values (P values < 0.001), but transiently increased to baseline levels after tracheal intubation.
180 s after tracheal intubation, there were no clinically important differences compared to baseline in heart rate, stroke volume index, or cardiac index. However, arterial pressure and systemic vascular resistance index remained well below baseline. 180 s after tracheal intubation, mean arterial pressure was 15 (10 to 20) mmHg lower than at baseline and it was < 65 mmHg in 21 patients (23%).
Supplemental Figure S1 shows Spaghetti plots for individual patients and Supplemental Figure S2 shows boxplots of changes in hemodynamic variables over time.
4 Discussion
In this prospective observational study, we sought to assess the relative contribution of various hemodynamic mechanisms to hypotension after induction of general anesthesia with sufentanil, propofol, and rocuronium in adults having non-cardiac surgery.
Heart rate and cardiac index increased after sufentanil administration, but presumably not due to a pharmacological effect of sufentanil. Instead, the increases likely reflect stress-induced sympathetic activation in anticipation of anesthetic induction. Propofol caused a clinically important reduction in arterial pressure. In addition, systemic vascular resistance index decreased significantly, by about 25%, after propofol administration. Heart rate returned to baseline after administration of propofol, and stroke volume index and cardiac index remained stable compared to baseline. Hypotension after propofol administration thus was linked to a decrease in systemic vascular resistance. Rocuronium administration had no additional clinically relevant effect on cardiovascular dynamics.
A controversy remains about propofol-induced post-induction hypotension. The main mechanisms proposed are a decrease in myocardial contractility, venous dilation with a decrease in venous return, and arterial dilation with a decrease in systemic vascular resistance [18,19,20]. Experimental and animal studies suggest that propofol reduces myocardial contractility. For example, propofol directly depresses myocardial contractility in isolated guinea pig myocardial trabeculae [26] and isolated perfused guinea pig hearts [27]. Propofol similarly reduces myocardial contractility in anesthetized rabbits [28]. Propofol decreases inotropy in anesthetized dogs, but also reduces arterial and venous vascular tone [29]. In 23 major abdominal surgery patients, propofol markedly decreased mean arterial pressure, heart rate, and cardiac output [17]. We found that neither stroke volume index nor cardiac index were reduced after propofol administration, suggesting that myocardial contractility was hardly influenced. Venous dilation has been proposed as a cause of propofol-induced hypotension [19, 30]. Venous dilation alone would reduce venous return to the heart, causing stroke volume to decrease. Since we did not observe a significant decrease in stroke volume index, propofol-induced venous dilation in our study seems unlikely. Our results thus suggest that propofol-induced post-induction hypotension results from arterial dilation with reduced systemic vascular resistance rather than venous dilation or reduced myocardial contractility. Our results are consistent with a previous small study which also reported decreased afterload without a compensatory increase in heart rate or cardiac output resulting in hypotension [31].
About a third of our patients had mean arterial pressures < 65 mmHg after propofol administration. While there is strong evidence that intraoperative hypotension is associated with postoperative organ failure and death [1,2,3,4,5,6,7] research only recently focused on characterizing different phases of intraoperative hypotension [9, 10]. For anesthesiologists it is crucial to acknowledge that about a third of all intraoperative hypotension occurs between anesthetic induction and surgical incision and that hypotension during this period appears equally harmful as hypotension that occurs during surgery [9]. Because post-induction hypotension is consequent to anesthetic drugs, much of it is presumably preventable—and probably should be prevented.
This reinforces the need to mitigate the potential cardiovascular effects of induction of general anesthesia. Our results indicate that post-induction hypotension results largely from arterial dilation, and therefore that vasopressors will generally be the most appropriate treatment. Which vasopressor(s) might be best remains unclear as there are sparse data related to the treatment or prophylaxis of post-induction hypotension by using vasopressors. In a preliminary study, phenylephrine and norepinephrine boluses effectively counteracted intraoperative hypotension caused by propofol anesthesia [32]. Although logic suggests that fluid loading may help prevent hypotension, pre-induction crystalloid loading does not prevent post-induction hypotension [33, 34]. Colloid loading may somewhat be more effective, but still fails to prevent much hypotension [35]. Vasopressors thus appear to be a preferable clinical strategy.
In our study, induction agents were standardized, but exact doses were not and remained at the discretion of the attending anesthesiologist. Additionally, we used a non-invasive finger-cuff method to assess advanced hemodynamic variables. The non-invasive monitoring system we used is well validated for the measurement of continuous blood pressure [21,22,23] and cardiac output [24, 25]. It is therefore unlikely that our overall conclusions would differ with invasive measurements. Further, we did not use echocardiography that could have provided important information on myocardial function. Our study was restricted to relatively young healthy adults and may thus not be generalizable to older and sicker patients, especially patients with cardiovascular co-morbidities.
5 Conclusions
In patients having non-cardiac surgery, anesthetic induction with sufentanil, propofol, and rocuronium was associated with a clinically important (and statistically significant) reduction in arterial pressure and systemic vascular resistance index. Heart rate and stroke volume index, and therefore cardiac index, basically remained stable during anesthetic induction. Post-induction hypotension therefore appears to result from arterial dilation with reduced systemic vascular resistance rather than venous dilation or reduced myocardial contractility. Future research should evaluate strategies for early detection and avoidance of post-induction hypotension, especially the (preemptive) use of vasopressors.
Data availability
Department of Anesthesiology, Center of Anesthesiology and Intensive Care Medicine, University Medical Center Hamburg-Eppendorf, Martinistrasse 52, 20246 Hamburg, Germany.
References
Sessler DI, Khanna AK. Perioperative myocardial injury and the contribution of hypotension. Intensive Care Med. 2018;44:811–22.
Walsh M, Devereaux PJ, Garg AX, Kurz A, Turan A, Rodseth RN, Cywinski J, Thabane L, Sessler DI. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology. 2013;119:507–15.
Sun LY, Wijeysundera DN, Tait GA, Beattie WS. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology. 2015;123:515–23.
Salmasi V, Maheshwari K, Yang D, Mascha EJ, Singh A, Sessler DI, Kurz A. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology. 2017;126:47–65.
Mascha EJ, Yang D, Weiss S, Sessler DI. Intraoperative mean arterial pressure variability and 30-day mortality in patients having noncardiac surgery. Anesthesiology. 2015;123:79–91.
Monk TG, Bronsert MR, Henderson WG, Mangione MP, Sum-Ping ST, Bentt DR, Nguyen JD, Richman JS, Meguid RA, Hammermeister KE. Association between intraoperative hypotension and hypertension and 30-day postoperative mortality in noncardiac surgery. Anesthesiology. 2015;123:307–19.
Stapelfeldt WH, Yuan H, Dryden JK, Strehl KE, Cywinski JB, Ehrenfeld JM, Bromley P. The SLUScore: a novel method for detecting hazardous hypotension in adult patients undergoing noncardiac surgical procedures. Anesth Analg. 2017;124:1135–52.
Sessler DI, Bloomstone JA, Aronson S, Berry C, Gan TJ, Kellum JA, Plumb J, Mythen MG, Grocott MPW, Edwards MR, Miller TE. Perioperative Quality Initiative consensus statement on intraoperative blood pressure, risk and outcomes for elective surgery. Br J Anaesth. 2019;122:563–74.
Maheshwari K, Turan A, Mao G, Yang D, Niazi AK, Agarwal D, Sessler DI, Kurz A. The association of hypotension during non-cardiac surgery, before and after skin incision, with postoperative acute kidney injury: a retrospective cohort analysis. Anaesthesia. 2018;73:1223–8.
Sudfeld S, Brechnitz S, Wagner JY, Reese PC, Pinnschmidt HO, Reuter DA, Saugel B. Post-induction hypotension and early intraoperative hypotension associated with general anaesthesia. Br J Anaesth. 2017;119:57–64.
Reich DL, Hossain S, Krol M, Baez B, Patel P, Bernstein A, Bodian CA. Predictors of hypotension after induction of general anesthesia. Anesth Analg. 2005;101:622–8.
Wierda JM, Schuringa M, van den Broek L. Cardiovascular effects of an intubating dose of rocuronium 06 mg kg-1 in anaesthetized patients, paralysed with vecuronium. Br J Anaesth. 1997;78:586–7.
Billard V, Moulla F, Bourgain JL, Megnigbeto A, Stanski DR. Hemodynamic response to induction and intubation Propofol/fentanyl interaction. Anesthesiology. 1994;81:1384–93.
Larsen R, Rathgeber J, Bagdahn A, Lange H, Rieke H. Effects of propofol on cardiovascular dynamics and coronary blood flow in geriatric patients. A comparison with etomidate. Anaesthesia. 1988;43(Suppl):25–31.
Haessler R, Madler C, Klasing S, Schwender D, Peter K. Propofol/fentanyl versus etomidate/fentanyl for the induction of anesthesia in patients with aortic insufficiency and coronary artery disease. J Cardiothorac Vasc Anesth. 1992;6:173–80.
Singh R, Choudhury M, Kapoor PM, Kiran U. A randomized trial of anesthetic induction agents in patients with coronary artery disease and left ventricular dysfunction. Ann Card Anaesth. 2010;13:217–23.
Moller Petrun A, Kamenik M. Bispectral index-guided induction of general anaesthesia in patients undergoing major abdominal surgery using propofol or etomidate: a double-blind, randomized, clinical trial. Br J Anaesth. 2013;110:388–96.
Goodchild CS, Serrao JM. Propofol-induced cardiovascular depression: science and art. Br J Anaesth. 2015;115:641–2.
Green DW. Cardiac output decrease and propofol: what is the mechanism? Br J Anaesth. 2015;114:163–4.
Kakazu CZ, Lippmann M. Playing with fire: debate about propofol-induced hypotension. Br J Anaesth. 2015;114:164–5.
Jeleazcov C, Krajinovic L, Munster T, Birkholz T, Fried R, Schuttler J, Fechner J. Precision and accuracy of a new device (CNAPTM) for continuous non-invasive arterial pressure monitoring: assessment during general anaesthesia. Br J Anaesth. 2010;105:264–72.
Wagner JY, Negulescu I, Schofthaler M, Hapfelmeier A, Meidert AS, Huber W, Schmid RM, Saugel B. Continuous noninvasive arterial pressure measurement using the volume clamp method: an evaluation of the CNAP device in intensive care unit patients. J Clin Monit Comput. 2015;29:807–13.
Smolle KH, Schmid M, Prettenthaler H, Weger C. The Accuracy of the CNAP(R) device compared with invasive radial artery measurements for providing continuous noninvasive arterial blood pressure readings at a medical intensive care unit: a method-comparison study. Anesth Analg. 2015;121:1508–16.
Wagner JY, Grond J, Fortin J, Negulescu I, Schofthaler M, Saugel B. Continuous noninvasive cardiac output determination using the CNAP system: evaluation of a cardiac output algorithm for the analysis of volume clamp method-derived pulse contour. J Clin Monit Comput. 2016;30:487–93.
Wagner JY, Korner A, Schulte-Uentrop L, Kubik M, Reichenspurner H, Kluge S, Reuter DA, Saugel B. A comparison of volume clamp method-based continuous noninvasive cardiac output (CNCO) measurement versus intermittent pulmonary artery thermodilution in postoperative cardiothoracic surgery patients. J Clin Monit Comput. 2018;32:235–44.
van Klarenbosch J, Stienen GJ, de Ruijter W, Scheffer GJ, de Lange JJ. The differential effect of propofol on contractility of isolated myocardial trabeculae of rat and guinea-pig. Br J Pharmacol. 2001;132:742–8.
Stowe DF, Bosnjak ZJ, Kampine JP. Comparison of etomidate, ketamine, midazolam, propofol, and thiopental on function and metabolism of isolated hearts. Anesth Analg. 1992;74:547–58.
Royse CF, Liew DF, Wright CE, Royse AG, Angus JA. Persistent depression of contractility and vasodilation with propofol but not with sevoflurane or desflurane in rabbits. Anesthesiology. 2008;108:87–93.
Pagel PS, Warltier DC. Negative inotropic effects of propofol as evaluated by the regional preload recruitable stroke work relationship in chronically instrumented dogs. Anesthesiology. 1993;78:100–8.
Muzi M, Berens RA, Kampine JP, Ebert TJ. Venodilation contributes to propofol-mediated hypotension in humans. Anesth Analg. 1992;74:877–83.
Claeys MA, Gepts E, Camu F. Haemodynamic changes during anaesthesia induced and maintained with propofol. Br J Anaesth. 1988;60:3–9.
Vallee F, Passouant O, Le Gall A, Joachim J, Mateo J, Mebazaa A, Gayat E. Norepinephrine reduces arterial compliance less than phenylephrine when treating general anesthesia-induced arterial hypotension. Acta Anaesthesiol Scand. 2017;61:590–600.
Turner RJ, Gatt SP, Kam PC, Ramzan I, Daley M. Administration of a crystalloid fluid preload does not prevent the decrease in arterial blood pressure after induction of anaesthesia with propofol and fentanyl. Br J Anaesth. 1998;80:737–41.
Khan AI, Fischer M, Pedoto AC, Seier K, Tan KS, Dalbagni G, Donat SM, Arslan-Carlon V. The impact of fluid optimisation before induction of anaesthesia on hypotension after induction. Anaesthesia. 2020;75:634–41.
Juri T, Suehiro K, Kuwata S, Tsujimoto S, Mukai A, Tanaka K, Yamada T, Mori T, Nishikawa K. Hydroxyethyl starch 130/0.4 versus crystalloid co-loading during general anesthesia induction: a randomized controlled trial. J Anesth. 2017;31:878–84.
Funding
Open Access funding enabled and organized by Projekt DEAL. CNSystems Medizintechnik GmbH (Graz, Austria) provided the technical equipment for the study. CNSystems was not involved in the collection of the data, drafting of the manuscript, or the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
BS conceived and designed the study, was responsible for data analysis and interpretation, drafted the manuscript, performed the statistical analyses, supervised the study. E-JB was responsible for acquisition of data, was responsible for data analysis and interpretation, critically revised the manuscript for important intellectual content. LB was responsible for data analysis and interpretation, critically revised the manuscript for important intellectual content. PH was responsible for data analysis and interpretation, critically revised the manuscript for important intellectual content. GG was responsible for data analysis and interpretation, critically revised the manuscript for important intellectual content. DY was responsible for data analysis and interpretation, performed the statistical analyses, drafted the manuscript. CM was responsible for data analysis and interpretation, performed the statistical analyses, critically revised the manuscript for important intellectual content. EJM was responsible for data analysis and interpretation, performed the statistical analyses, critically revised the manuscript for important intellectual content. DIS was responsible for data interpretation, drafted the manuscript. DER conceived and designed the study, was responsible for acquisition of data, was responsible for data analysis and interpretation, drafted the manuscript. All authors read and approved the final version of the manuscript and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
Bernd Saugel has received institutional restricted research grants, honoraria for giving lectures, and refunds of travel expenses from CNSystems Medizintechnik GmbH (Graz, Austria). Bernd Saugel has received honoraria for consulting, honoraria for giving lectures, and refunds of travel expenses from Edwards Lifesciences Inc. (Irvine, CA, USA). Bernd Saugel has received honoraria for consulting, institutional restricted research grants, honoraria for giving lectures, and refunds of travel expenses from Pulsion Medical Systems SE (Feldkirchen, Germany). Bernd Saugel has received institutional restricted research grants from Retia Medical LLC. (Valhalla, NY, USA). Bernd Saugel has received honoraria for giving lectures from Philips Medizin Systeme Böblingen GmbH (Böblingen, Germany). Bernd Saugel has received honoraria for consulting, institutional restricted research grants, and refunds of travel expenses from Tensys Medical Inc. (San Diego, CA, USA). Daniel I. Sessler is a consultant for Edwards Lifesciences Inc and Sensifree (Cupertino, CA, USA). The Department of Outcomes Research conducts research funded by Edwards Lifesciences Inc.. All other authors have no conflicts of interest.
Consent to participate
All patients provided written informed consent.
Consent to publish
All patients signed informed consent regarding publishing their data.
Ethical approval
The study was approved by the Ethics Committee of the Medical Association of Hamburg, Hamburg, Germany (Chairperson Prof. R. Stahl) on January 9, 2018.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10877_2021_653_MOESM1_ESM.tif
Hemodynamic variables during the induction of general anesthesia in individual patients. Spaghetti plots are showing the changes in hemodynamic variables during the induction of general anesthesia in individual patients. Black and blue lines therefore show individual trajectories of hemodynamic variables over time. MAP, mean arterial pressure; SAP, systolic arterial pressure; DAP, diastolic arterial pressure; SVRI, systemic vascular resistance index; HR, heart rate; SVI, stroke volume index; CI, cardiac index. Supplementary material 1 (TIF 32885 kb)
10877_2021_653_MOESM2_ESM.tif
Hemodynamic variables during the induction of general anesthesia (change from baseline). Boxplots showing the change of hemodynamic variables during the induction of general anesthesia compared to baseline. Boxplots show mean (triangle) and median (horizontal bar) with 25th-75th percentile (box). Whiskers extend to the most extreme observations within 1.5 times the interquartile range of the first and third quartiles, respectively. Circles represent outliers. MAP, mean arterial pressure; SAP, systolic arterial pressure; DAP, diastolic arterial pressure; SVRI, systemic vascular resistance index; HR, heart rate; SVI, stroke volume index; CI, cardiac index. Supplementary material 2 (TIF 12696 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saugel, B., Bebert, EJ., Briesenick, L. et al. Mechanisms contributing to hypotension after anesthetic induction with sufentanil, propofol, and rocuronium: a prospective observational study. J Clin Monit Comput 36, 341–347 (2022). https://doi.org/10.1007/s10877-021-00653-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-021-00653-9