Abstract
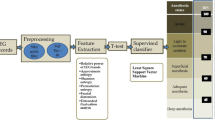
Monitoring level of hypnosis is a major ongoing challenge for anesthetists to reduce anesthetic drug consumption, avoiding intraoperative awareness and prolonged recovery. This paper proposes a novel automated method for accurate assessing of the level of hypnosis with sevoflurane in 17 patients using the electroencephalogram signal. In this method, a set of distinctive features and a hierarchical classification structure based on support vector machine (SVM) methods, is proposed to discriminate the four levels of anesthesia (awake, light, general and deep states). The first stage of the hierarchical SVM structure identifies the awake state by extracting Shannon Permutation Entropy, Detrended Fluctuation Analysis and frequency features. Then deep state is identified by extracting the sample entropy feature; and finally light and general states are identified by extracting the three mentioned features of the first step. The accuracy of the proposed method of analyzing the brain activity during anesthesia is 94.11%; which was better than previous studies and also a commercial monitoring system (Response Entropy Index).



Similar content being viewed by others
References
Monk TG, Saini V, Weldon BC, Sigl JC. Anesthetic management and one-year mortality after noncardiac surgery. Anesth Analg. 2005;100:4–10.
Sebel PS, Bowdle TA, Ghoneim MM, Rampil IJ, Padilla RE, Gan TJ, Domino KB. The incidence of awareness during anesthesia: a multicenter United States study. Anesth Analg. 2004;99:833–9.
Gugino LD, Chabot RJ, Prichep LS, John ER, Formanek V, Aglio LS. Quantitative EEG changes associated with loss and return of consciousness in healthy adult volunteers anaesthetized with propofol or sevoflurane. Br J Anaesth. 2001;87:421–8.
Viertio-Oja H, Maja V, Sarkela M, Talja P. Description of the entropy algorithm as applied in the Datex-Ohmeda S/5 entropy module. Acta Anaesthesiol Scand. 2004;48:154–61.
Rampil IJ. A primer for EEG signal processing in anesthesia. Anesthesiology. 1998;89:980–1002.
Kreuer S, Wilhelm W. The narcotrend monitor. Best Practice Res Clin Anaesth. 2006;20:111–9.
Kortelainen J, Väyrynen E, Seppänen T. Depth of anesthesia during multidrug infusion: separating the effects of propofol and remifentanil using the spectral features of EEG. IEEE Trans Biomed Eng. 2011;58:1216–23.
Nguyen-Ky T, Wen P, Li Y, Gray R. Measuring and reflecting depth of anesthesia using wavelet and power spectral density. IEEE Trans Inf Technol Biomed. 2011;15:630–9.
Lalitha V, Eswaran C. Automated detection of anesthetic depth levels using chaotic features with artificial neural networks. J Med Syst. 2007;31:445–52.
Zhang XS, Roy RJ, Jensen EW. EEG complexity as a measure of depth of anaesthesia for patients. IEEE Trans Biomed Eng. 2001;48:1424–33.
Nguyen-Ky T, Wen P, Li Y. Consciousness and depth of anesthesia assessment based on bayesian analysis of EEG signals. IEEE Trans Biomed Eng. 2013;60:1488–98.
Nguyen-Ky T, Wen P, Li Y. Monitoring the depth of anaesthesia using Hurst exponent and Bayesian methods. IET Signal Proc. 2014;8:907–17.
Liu Q, Chen YF, Fan SZ, Abbod M, Shieh JS. Quasi-periodicities detection using phase-rectified signal averaging in EEG signals as a depth of anesthesia monitor. IEEE Trans Neural Syst Rehabil Eng. 2017;25:1773–84.
Kuhlmann L, et al. tracking electroencephalographic changes using distributions of linear models: application to propofol-based depth of anesthesia monitoring. IEEE Trans Biomed Eng. 2017;64:870–81.
Shalbaf R, Behnam H, Sleigh JW, Voss LJ. Using the Hilbert-Huang transform to measure the electroencephalographic effect of propofol. Physiol Meas. 2012;33:271–85.
Shalbaf R, Behnam H, Sleigh JW, Steyn-Ross DA, SteynRoss ML. Frontal-temporal synchronization of EEG signals quantified by order patterns cross recurrence analysis during propofol anesthesia. IEEE Trans Neural Syst Rehabil Eng. 2015;23:468–74.
Gifani P, Rabiee HR, Hashemi MH, Zadeh MS, Taslimi P, Ghanbari M. Optimal fractal-scaling analysis of human EEG dynamic for depth of anesthesia quantification. J Franklin Inst. 2007;344:212–29.
Jospin M, Caminal P, Jensen EW, et al. Detrended fluctuation analysis of EEG as a measure of depth of anaesthesia. IEEE Trans Biomed Eng. 2007;54:840–6.
Ferenets R, Lipping T, Anier A, Jantti V, Melto S, Hovilehto S. Comparison of entropy and complexity measures for the assessment of depth of a sedation. IEEE Trans Biomed Eng. 2006;53:1067–77.
Shalbaf R, Behnam H, Sleigh JW, Voss LJ. Measuring the effects of sevoflurane on electroencephalogram using sample entropy. Acta Anaesthesiol Scand. 2012;56:880–9.
Shalbaf R, Behnam H, Sleigh JW, Steyn-Ross A, Voss LJ. Monitoring the depth of anesthesia using entropy features and an artificial neural network. J Neurosci Methods. 2013;218:17–24.
Liang Z, Wang Y, Sun X, Li D, Voss LJ, Sleigh JW, Hagihira S, Li X. EEG entropy measures in anesthesia. Front Comput Neurosci. 2015;18:9–16.
Mirsadeghi M, Behnam H, Shalbaf R, Jelveh Moghadam H. Characterizing awake and anesthetized states using a dimensionality reduction method. J Med Syst. 2016;40:13.
Chen D, Li D, Xiong M, Bao H, Li X. GPGPU-aided ensemble empirical-mode decomposition for EEG analysis during anesthesia. IEEE Trans Inf Technol Biomed. 2010;14:1417–27.
Kortelainen J, Väyrynen E, Seppänen T. Isomap approach to EEG-based assessment of neurophysiological changes during anesthesia. IEEE Trans Neural Syst Rehabil Eng. 2011;19:113–20.
Shalbaf R, Behnam H, Jelveh Moghadam H. Monitoring depth of anesthesia using combination of EEG measure and hemodynamic variables. Cogn Neurodyn. 2014;8:1–11.
Li T, Wen P. Depth of anaesthesia assessment using interval second-order difference plot and permutation entropy techniques. IET Signal Proc. 2017;11:221–7.
Zhang XS, Roy RJ. Derived fuzzy knowledge model for estimating the depth of anesthesia. IEEE Trans Biomed Eng. 2001;48(3):312–23.
Shalbaf A, Saffar M, Sleigh JW, Shalbaf R. Monitoring the depth of anesthesia using a new adaptive neuro-fuzzy system. IEEE J Biomed Health Inf. 2018;22:671–7.
Esmaeili V, Assareh A, Shamsollahi MB, Moradi MH, Arefian NM. Estimating the depth of anesthesia using fuzzy soft computation applied to EEG features. Intell Data Anal. 2008;12:393–407.
Sela Y, Freiman M, Dery E, Edrei Y, Safadi R, Pappo O, Joskowicz L, Abramovitch R. fMRI-based hierarchical SVM model for the classification and grading of liver fibrosis. IEEE Trans Biomed Eng. 2011;58:2574–81.
Sekar BD, Chui Dong M, Shi J, Hu XY. Fused hierarchical neural networks for cardiovascular disease diagnosis. IEEE Sens J. 2012;12:644–50.
Selver MA, Akay O, Ardali E, Yavuz AB, Onal O, Ozden G. Cascaded and hierarchical neural networks for classifying surface images of marble slabs. IEEE Trans Syst Man Cybern. 2009;39:426–39.
Li Y, Du L, Liu H. Hierarchical classification of moving vehicles based on empirical mode decomposition of micro-doppler signatures. IEEE Trans Geosci Remote Sens. 2013;51:3001–13.
Vanitha L, Suresh GR (2014) Hierarchical SVM to detect mental stress in human beings using Heart Rate Variability. In: 2nd international conference on devices, circuits and systems, pp 1–5
Acir N, Oztura I, Kuntalp M, Baklan B, Guzelis C. Automatic detection of epileptiform events in EEG by a three-stage procedure based on artificial neural networks. IEEE Trans Biomed Eng. 2005;52(1):30–40.
Chen K. On the use of different speech representations for speaker modeling. IEEE Trans Syst Man Cybern C. 2005;35(3):301–14.
Chen K. A connectionist method for pattern classification with diverse features. Pattern Recognit Lett. 1998;19(7):545–58.
Mckay ID, Voss LJ, Sleigh JW, Barnard JP, Johannsen EK. Pharmacokinetic pharmacodynamic modeling the hypnotic effect of sevoflurane using the spectral entropy of the electroencephalogram. Anesth Analg. 2006;102:91–7.
Chander D, García PS, MacColl JN, Illing S, Sleigh JW. Electroencephalographic variation during end maintenance and emergence from surgical anesthesia. PLoS ONE. 2014;9(9):e106291.
Richman JS, Moorman JR. Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol. 2000;278:121–8.
Bandt C, Pompe B. Permutation entropy: a natural complexity measure for time series. Phys Rev Lett. 2002;88:174102.
Bertsekas DP. Nonlinear programming. Belmont, MA: Athenas Scientific; 1995.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study received ethics committee approval at Waikato Hospital, New Zealand.
Informed consent
Informed written consent was obtained from all patients included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shalbaf, A., Shalbaf, R., Saffar, M. et al. Monitoring the level of hypnosis using a hierarchical SVM system. J Clin Monit Comput 34, 331–338 (2020). https://doi.org/10.1007/s10877-019-00311-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-019-00311-1