Abstarct
To investigate the use of two-site regional oxygen saturations (rSO2) and end tidal carbon dioxide (EtCO2) to assess the effectiveness of resuscitation and return of spontaneous circulation (ROSC). Eight mechanically ventilated juvenile swine underwent 28 ventricular fibrillatory arrests with open cardiac massage. Cardiac massage was administered to achieve target pulmonary blood flow (PBF) as a percentage of pre-cardiac arrest baseline. Non-invasive data, including, EtCO2, cerebral rSO2 (C-rSO2) and renal rSO2 (R-rSO2) were collected continuously. Our data demonstrate the ability to measure both rSO2 and EtCO2 during CPR and after ROSC. During resuscitation EtCO2 had a strong correlation with goal CO with r = 0.83 (p < 0.001) 95% CI [0.67–0.92]. Both C-rSO2 and R-rSO2 had moderate and statistically significant correlation with CO with r = 0.52 (p = 0.003) 95% CI (0.19–0.74) and 0.50 (p = 0.004) 95% CI [0.16–0.73]. The AUCs for sudden increase of EtCO2, C-rSO2, and R-rSO2 at ROSC were 0.86 [95% CI, 0.77–0.94], 0.87 [95% CI, 0.8–0.94], and 0.98 [95% CI, 0.96–1.00] respectively. Measurement of continuous EtCO2 and rSO2 may be used during CPR to ensure effective chest compressions. Moreover, both rSO2 and EtCO2 may be used to detect ROSC in a swine pediatric ventricular fibrillatory arrest model.
Similar content being viewed by others
1 Introduction
Cardiac arrest occurs in 2–6% of children admitted to Pediatric Intensive Care Units [1, 2]. The overall survival for pediatric patients with in-hospital cardiac arrest remains low at less than 40% [3, 4]. Although severe hypoxia and respiratory failure remain the most common cause of pediatric in hospital cardiac arrests, 10–15% have rhythm that requires defibrillation [5]. The outcome of cardiac arrest is dependent on critical interventions, especially early defibrillation, effective chest compressions and assisted ventilation [6, 7]. Invasive blood pressure and cardiac output monitoring provide an accurate means of assessing the effectiveness of chest compressions [8]. However, invasive monitoring at the time of either the arrest or initiation of resuscitation occurs in less than 50% of in-hospital pediatrics cardiac arrest cases and almost never occurs in out-of-hospital pediatric cardiac arrest cases [2]. The need for accurate, non-invasive, and real-time monitoring of both oxygenation and circulation during resuscitation is, therefore, paramount to the improvement of resuscitation outcomes.
Despite its limitations, end-tidal carbon dioxide (EtCO2) monitoring remains the only noninvasive tool that has been used and advocated during CPR as a marker of effectiveness of resuscitation [9]. Furthermore, EtCO2 has been suggested to correlate with cardiac output and cerebral perfusion, and it has also been reported to be a predictor of survival and prognosis in patients after cardiac arrest and resuscitation [10, 11]. However, measurement of EtCO2 can be particularly unreliable in low-flow states such as during CPR or in the presence of significant pulmonary pathology. Moreover, the accuracy of EtCO2 measurement depends on proper capnography waveforms that may be difficult to obtain during chest compressions [12, 13]. Finally, EtCO2 does not provide any meaningful information about the quality of organ perfusion and oxygenation during resuscitation [14].
Regional oxygen saturation monitoring with near infrared spectroscopy (NIRS) has emerged as a noninvasive surrogate monitor of regional oxygen saturation (rSO2) [15, 16]. During critical illness, NIRS probes are routinely placed on the forehead to measure cerebral rSO2 (C-rSO2) and the back to measure renal rSO2 (R-rSO2), referred to as two-site rSO2. Two-site rSO2 can provide a real-time indicator of the balance between oxygen supply and demand in the intraoperative and intensive care setting. Moreover, two-site rSO2 represent two opposite poles of circulation, and the compensatory response to low cardiac output can be reflected in a decrease in R-rSO2 while C-rSO2 is preserved. This clinical information can help the provider to make treatment decisions and assess the effect of therapeutic interventions in settings of low cardiac output, such as cardiogenic, hemorrhagic and septic shock [17]. Moreover, NIRS does not require pulsatile flow, which makes it an ideal monitoring tool during cardiac arrest [18]. Although NIRS technology has been validated and used in many clinical scenarios, limited studies have examined the role of two-site rSO2 monitoring during cardiac arrest to assess the effectiveness of chest compressions and detection of ROSC without interrupting resuscitation efforts [19,20,21,22].
2 Materials and methods
All applicable international, national, and institutional guidelines for the care of experimental animals for scientific purposes were applied throughout the study; the experimental protocol was approved by University of Wisconsin Institutional Animal Care and Use Committee, in accordance with the guidelines in the US Department of Health and Human Services and the National Institutes for Health. The choice of domestic pigs was based on the fact that the porcine heart closely resembles the human heart from the point of view of size, physiology and anatomy.
2.1 Animal preparation
This study was performed in eight healthy 2- to 3-month-old mixed-breed domestic swine (Sus scrofa) with weight of 23 ± 3 kg. Animals had free access to water but were fasted overnight. Each animal was pre-medicated with intramuscular telazol (4–7 mg/kg) and xylazine (2 mg/kg). Subsequently, a 20 g intravenous catheter was placed into an ear vein for additional anaesthetic agents (Propofol, 2–10 mg/kg) prior to the procedure. The animal was intubated with cuffed endotracheal tube and mechanically ventilated under general anaesthesia using mixture of room air and titrated isoflurane (0.5–1.5% inspired concentration). Volumetric capnography monitor (Philips, Carlsbad, CA) was connected to the ventilator circuit via a side stream capnostat sensor placed at the proximal end of the endotracheal tube (between the endotracheal tube and the ventilator circuit). Then animals were deeply sedated with adequate pain control throughout the experiment using fentanyl (3.0–10.0 μg/kg/h IV) as needed. The tidal volume was initially set at 8–10 mL/kg and the ventilator rate at 12 breaths per minute; ventilator settings then were adjusted to maintain PaCO2 at 35 to 45 mm Hg. The femoral artery was accessed by percutaneous methods then a 6 Fr sheath was placed and advanced to the abdominal aorta to measure systemic pressure and to withdraw blood for blood gas analysis. A 6 Fr sheath was also placed in the femoral vein by percutaneous methods for further administration of medications. A median sternotomy was performed and the heart was exposed. Finally, an ultrasonic flow probe (PAU Series, ADInstruments, Dunedin, New Zealand) was placed around the pulmonary artery.
Following surgical procedures, adhesive, non-invasive NIRS probes were placed on a shaved portion of the left forehead scalp laterally from the midline, and left somatic (flank) region above the kidney. These locations were selected to reflect the locations used for clinical C-rSO2 and R-rSO2 monitoring in children. Then probes were connected to a Somanetics Invos 5100C Monitor (OxyAlertTM, Somanetics Invos 5100C, Somanetics Corporation, Troy, MI, USA). Baseline C-rSO2 and R-rSO2 readings were continuously obtained before VF induction and then was continuously monitored and recorded throughout the entirety of the experimental protocol.
Continuous monitoring included ECG, peripheral oxygen saturation, invasive arterial blood pressure monitoring, cerebral and renal saturation by near infrared spectroscopy (INVOS® Cerebral/Somatic Oximeter monitor, Somanetics, Troy, MI, USA), and the respiratory volumes, pressures, EtCO2, volume of carbon dioxide monitoring, and tidal volume and dead space ratio by means of a volumetric capnography monitor. Blood gases, lactate, and hemoglobin were analyzed using a blood gas analyzer (pHOx Basics; Nova Biomedical, Waltham, MA).
2.2 Study protocol
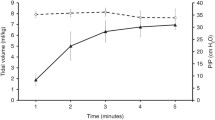
Following baseline data acquisition, ventricular fibrillation was induced with current from a 9 V battery applied to the epicardial surface of the right ventricle for 3 s. Examination of the ECG tracing, absence of contractility, and the absence of pulses in the arterial pressure waveform was used to confirm VF. One minute following loss of pulse pressure, CPR was initiated by open manual cardiac massage. Cardiac massage was administered and was altered every 2 min during resuscitation to achieve target pulmonary blood flow (PBF) as a percentage of pre-cardiac arrest baselines (30%, 50%, and 70% of prearrest mean PBF) until ROSC or euthanasia. Non-invasive and invasive hemodynamic data was continuously monitored and collected through the whole experiment. Cardiac massage was at a rate of approximately 100 cycles per minute. Epinephrine, lidocaine, and defibrillation was administered per Pediatric Advanced Life Support (PALS) guidelines (Fig. 1). If there was no ROSC, the cycle was repeated per PALS protocol. ROSC was defined as restoration of perfusing rhythm, arterial systolic blood pressure of at least 50 mmHg and pulse pressure of 20 mmHg lasting at least 1 min. If there is no ROSC after 15 min resuscitation was discontinued.
After successful resuscitation, the animals was maintained on mechanical ventilation and continuous monitoring. Blood gases were obtained, metabolic acidosis was treated with normal saline and/or sodium bicarbonate and the ventilator was adjusted to achieve normal ventilation (PaCO2 = 35–45 mmHg). Normal saline boluses were administered to treat hypotension. After 30 min of stability, the experiment was repeated in the animal up to four additional times.
2.3 Statistical analysis
Hemodynamic variables and levels of blood gases were summarized in terms of means ± standard deviations, stratified by event type. The correlations between measured cardiac output and rSO2 and EtCO2 parameters were evaluated using the nonparametric Spearman’s rank correlation analysis. Receiver operating characteristics (ROC) curve analysis was conducted to evaluate whether cerebral, renal or EtCO2 can predict ROSC. The strength of the prediction is quantified by calculating the area under the curve (AUC) which was reported along with the corresponding 95% confidence intervals. The Youden criterion was used to determine optimal threshold cutoff threshold for predicting ROSC [23]. Linear mixed effects modeling with animal-specific random effects was utilized when comparing outcome parameters between goal cardiac outputs. All reported P-values are two-sided and P < 0.05 was used to define statistical significance. Statistical analysis was conducted using SAS software (SAS Institute Inc., Cary NC), version 9.4.
3 Results
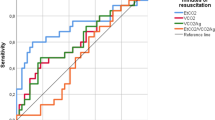
A total of 8 mechanically ventilated juvenile swine underwent 28 VF arrests with open cardiac massage (5 animals underwent 4 VF arrests, 2 underwent 3 VF arrests, and one animal underwent only 2 VF arrests). Hemodynamic variables and blood gases before cardiac arrest, during CPR and after ROSC are shown in Table 1. With onset of cardiac arrest, there was a sudden (within 30 s) decrease in EtCO2, R-rSO2 and C-rSO2 by 17.1%, 20.4%, and 24.3% respectively. Correlations between R-rSO2, C-rSO2, and EtCO2 before cardiac arrest, and after ROSC are shown in Table 2. There was a strong and statistically significant correlation between EtCO2 and cardiac output after ROSC with r = 0.74 (p = 0.03) 95% CI [0.08–0.92]. During resuscitation, EtCO2 had a strong correlation with goal CO, measured as a percentage of baseline PBF, with correlation coefficient of r = 0.83 (p < 0.001) 95% CI [0.67–0.92]. Both C-rSO2 and R-rSO2 had moderate and statistically significant correlation with measured CO with correlation coefficient of 0.52 (p = 0.003) 95% CI (0.19–0.74) and 0.50 (p = 0.004) 95% CI [0.16–0.73]. There were no significant differences detected in C-rSO2, R-rSO2 or EtCO2 levels when goal cardiac output was increased from 50 to 70% (Fig. 2). The AUCs for sudden increase in EtCO2, C-rSO2, and R-rSO2 at ROSC were 0.86 [95% CI, 0.77–0.94], 0.87 [95% CI, 0.8–0.94], and 0.98 [95% CI, 0.96–1.00] respectively (Fig. 3). The optimal threshold for percentage change cutoffs for EtCO2, C-rSO2, and R-rSO2 were 9.3%, 4.0%, and 5.0% respectively. At these cutoffs, a sudden increase in EtCO2, C-rSO2, and R-rSO2 had similar sensitivities (96%, 92%, and 96%). However, R-rSO2 was the most specific when compared to EtCO2 and C-rSO2 (91% vs. 73% and 72%). Further analysis included only the first VF arrest for each animal showed that AUCs for sudden increase in EtCO2, C-rSO2, and R-rSO2 at ROSC were 0.86 [95% CI 0.82–0.90], 0.72 [95% CI 0.66–0.78], and 0.84 [95% CI 0.79–0.89] respectively (Fig. 3). The optimal threshold for percentage change cutoffs for EtCO2, C-rSO2, and R-rSO2 were 6.8%, 9.9%, and 7.5% respectively. At these cutoffs, a sudden increase in EtCO2 was more sensitive than C-rSO2 and R-rSO2 (93% vs. 50% and 82%). However, C-rSO2 and R-rSO2 were more specific when compared to EtCO2 (93% and 82% vs. 76%) (Fig. 4).
4 Discussion
Published guidelines for CPR increased the focus on ways to ensure that high-quality CPR is performed in all resuscitation attempts to improve outcomes from cardiac arrest [24, 25]. Recent adult studies have assessed the usefulness of EtCO2 and C-rSO2 as a guide to resuscitative efforts during CPR [26,27,28], yet there have been no studies that assessed the use of R-rSO2, nor the role of two-site rSO2 in assessing quality of resuscitation or ROSC. We report the potential use of two-site rSO2, and EtCO2 to monitor the effectiveness and quality of CPR, and ROSC. This is the first study to evaluate the relationship between changes in C-rSO2, R-rSO2, and EtCO2 and CO during resuscitation and ROSC in swine pediatric VF arrest model.
In this study we demonstrate that EtCO2 and two-site rSO2 can be used to assess and guide effectiveness of chest compressions especially when resuscitation efforts are suboptimal. As EtCO2 reflects PBF, and two-site rSO2 reflects the balance between oxygen delivery and extraction at 2 two different vascular beds [28], the use of both monitoring devices during resuscitation may provide complementary information on the quality of resuscitation organ perfusion, and tissue oxygenation during CPR. Our result is consistent with the findings of Koyama et al. who reported that C-rSO2 could reliably assess the quality of chest compressions and improve effectiveness of CPR in adult cardiac arrest patients. However, in that study only C-rSO2 waveform (not the absolute value) was used to assess the quality of chest compression [26]. In our study the quality of chest compressions was controlled and continuously monitored and the absolute rSO2 was monitored as well. In contrast to our results, Kämäräinen et al. found that C-rSO2 remained low during high-quality resuscitation and that improvement in CPR was not significantly reflected in C-rSO2 [27]. However, the assessment of C-rSO2 was not monitored prior to initiation of CPR and therefore the C-rSO2 before cardiac arrest was not known. Moreover, quality of CPR provided was monitored using defibrillator with CPR quality analysis features and no other direct or invasive measurements of CPR quality was measured. In regard to EtCO2, a recent study using a lamb asphyxial cardiac arrest model, Chandrasekharan et al. demonstrated a relationship between EtCO2 and adequate chest compression during resuscitation, and concluded that a rapid increase in EtCO2 with a threshold of > 32 mmHg is 100 sensitive and 97% specific in predicting ROSC [29]. Moreover, a recent multicenter study of 583 adult patients with in- and out hospital CA showed that depth of chest compression was associated with higher EtCO2 which suggest that EtCO2 monitoring during CPR might be a useful tool to guide effective resuscitation after CA [30].
Our data also suggest that both rSO2 and EtCO2 can detect ROSC during CPR without interrupting chest compressions. Although C-rSO2, R-rSO2, and EtCO2 were all very sensitive tests to detect ROSC, R-rSO2 was more specific than both C-rSO2 and EtCO2. Thus adding renal NIRS monitoring during resuscitation might not only guide resuscitation, but can be used to detect with more confidence ROSC. Our results is consistent with Pokorna et al. who showed that a sudden increase in EtCO2 > 10 mmHg during out-of-hospital CPR could be used as an early indicator of ROSC [31]. Similarly, with the findings of Singer et al. who reported that during outside hospital CA, both mean C-rSO2 and EtCO2 during CPR can be used to predict ROSC [32]. They found that EtCO2 was more sensitive and C-rSO2 was more specific at predicting ROSC. However, they didn’t assess the sensitivity and specificity of sudden increase in both to detect ROSC. Moreover, a growing number of recent studies demonstrated that a higher mean cerebral rSO2 during CPR was observed in patients who achieved ROSC compared to non-survivors [14, 20, 32, 33]. We are not aware of any studies that assessed the ability of two-site rSO2 to detect ROSC during CPR.
Finally, our results demonstrated that both rSO2 and EtCO2 rapidly decline after loss of pulse pressure. This is consistent with the findings of Reynolds et al. who reported that forelimb rSO2 in a porcine VF arrest model rapidly declined by 28% in 1 min after loss of pulse pressure. However, they reported that EtCO2 did not drop immediately [34]. Similarly, Putzer et al. found that C-rSO2 decreased during periods of untreated cardiac arrest in an animal model of hypothermic cardiac arrest [35]. The recognition of acute sudden drop in rSO2 and EtCO2 in a hemodynamically unstable patient could be extremely vital in the recognition of cardiac arrest and early initiation of resuscitation efforts especially in patients who lack invasive lines.
Our study has obvious limitations. First, our study included animals with open chest without sufficient thoracic pump function, which may have affected venous return and cardiac output. We acknowledge that our model might not resemble the clinical situation of most critically ill patients who suffer cardiac arrest and require CPR, however, it might be clinically relevant in anaesthetized patients or patients who just undergone cardiac surgery and suffer a sudden, cardiac deterioration and arrest. Second, the cardiac massage was performed by hand and mean PBF goal was used during resuscitation. Also animals received normal saline and/or sodium bicarbonate after ROSC which might led to a transient increase in EtCO2 without an actual increase in cardiac output during subsequent arrests, however, further analysis of the first VF arrest didn’t indicate such effect, in the contrary, EtCO2 was more sensitive than C-rSO2 and R-rSO2 to detect ROSC during the first VF arrests when compared to subsequent arrests (Fig. 4). Third, all animals had relatively healthy lungs, which may not reflect common pediatric cardiac arrests. This latter point presents a concern as EtCO2 is affected by alveolar dead space, which may be a confounding factor when assessing changes in CO. Finally, analgosedative drugs were administered, as necessary, to maintain adequate analgesia and anesthesia. The use of these drugs may have affected cardiovascular function and autonomic control during resuscitation.
5 Conclusions
In this swine pediatric VF arrest model, two-site rSO2 obtained by NIRS technology, and EtCO2 correlates with cardiac output changes during CPR and can be used to guide resuscitations efforts and detect ROSC without interrupting resuscitation efforts. Further studies are required to explore the use of two-site rSO2 and capnography as a tool for early detection of CA, monitor effectiveness of chest compression, and detection of ROSC among critically ill pediatric patients who suffer from cardiac arrest.
References
Girotra S, Spertus JA, Li Y, Berg RA, Nadkarni VM, Chan PS, et al. Survival trends in pediatric in-hospital cardiac arrests: an analysis from Get With the Guidelines-Resuscitation. Circ Cardiovasc Qual Outcomes. 2013;6(1):42–9. https://doi.org/10.1161/CIRCOUTCOMES.112.967968.
Nadkarni VM, Larkin GL, Peberdy MA, Carey SM, Kaye W, Mancini ME, et al. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA 2006;295(1):50–7. https://doi.org/10.1001/jama.295.1.50.
Yam N, McMullan DM. (2017) Extracorporeal cardiopulmonary resuscitation. Ann Transl Med. 5(4):72.
de Caen AR, Berg MD, Chameides L, Gooden CK, Hickey RW, Scott HF, et al. Part 12: pediatric advanced life support: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 Suppl 2):526–42. https://doi.org/10.1161/CIR.0000000000000266.
Hunt EA, Duval-Arnould JM, Bembea MM, Raymond T, Calhoun A. Association between time to defibrillation and survival in pediatric in-hospital cardiac arrest with a first documented shockable rhythm. JAMA Netw Open. 2018;1(5):e182643. https://doi.org/10.1001/jamanetworkopen.2018.2643.
Jacobs I, Nadkarni V, Arrest tITFoC, Outcomes CR, PARTICIPANTS C, Bahr J. Cardiac arrest and cardiopulmonary resuscitation outcome reports. Circulation 2004;110(21):3385–97. https://doi.org/10.1161/01.cir.0000147236.85306.15.
Cunningham LM, Mattu A, O’Connor RE, Brady JW. Cardiopulmonary resuscitation for cardiac arrest: the importance of uninterrupted chest compressions in cardiac arrest resuscitation. Am J Emerg Med. 2012;30(8):1630–8. https://doi.org/10.1016/j.ajem.2012.02.015.
Perkins GD, Jacobs IG, Nadkarni VM, Berg RA, Bhanji F, Biarent D, et al. Cardiac Arrest and Cardiopulmonary Resuscitation Outcome Reports: Update of the Utstein Resuscitation Registry Templates for Out-of-Hospital Cardiac Arrest: A Statement for Healthcare Professionals From a Task Force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia); and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Resuscitation 2015;96:328–40. https://doi.org/10.1016/j.resuscitation.2014.11.002.
Eckstein M, Hatch L, Malleck J, McClung C, Henderson SO. End-tidal CO2 as a predictor of survival in out-of-hospital cardiac arrest. Prehosp Disaster Med. 2011;26(3):148–50.
Lewis LM, Stothert J, Standeven J, Chandel B, Kurtz M, Fortney J. Correlation of end-tidal CO2 to cerebral perfusion during CPR. Ann Emerg Med. 1992;21(9):1131–4.
Morisaki H, Takino Y, Kobayashi H, Ando Y, Ichikizaki K. End-tidal carbon dioxide concentration during cardiopulmonary resuscitation in patients with pre-hospital cardiac arrest. Masui 1991;40(7):1048–51.
Thompson JE, Jaffe MB. Capnographic waveforms in the mechanically ventilated patient. Respir Care. 2005;50(1):100–8; discussion 108–109.
Trillo G, von Planta M, Kette F. ETCO2 monitoring during low flow states: clinical aims and limits. Resuscitation 1994;27(1):1–8.
Ahn A, Nasir A, Malik H, D’Orazi F, Parnia S. A pilot study examining the role of regional cerebral oxygen saturation monitoring as a marker of return of spontaneous circulation in shockable (VF/VT) and non-shockable (PEA/Asystole) causes of cardiac arrest. Resuscitation. 2013;84(12):1713–6. https://doi.org/10.1016/j.resuscitation.2013.07.026.
Ricci M, Lombardi P, Schultz S, Galindo A, Coscarella E, Vasquez A, Rosenkranz E. Near-infrared spectroscopy to monitor cerebral oxygen saturation in single-ventricle physiology. J Thorac Cardiovasc Surg. 2006;131(2):395–402. https://doi.org/10.1016/j.jtcvs.2005.07.039.
Hansen JH, Schlangen J, Armbrust S, Jung O, Scheewe J, Kramer HH. Monitoring of regional tissue oxygenation with near-infrared spectroscopy during the early postoperative course after superior cavopulmonary anastomosis. Eur J Cardiothorac Surg. 2013;43(2):e37–43. https://doi.org/10.1093/ejcts/ezs581.
Al-Subu AM, Rehder KJ, Cheifetz IM, Turner DA. Non invasive monitoring in mechanically ventilated pediatric patients. Expert Rev Respir Med. 2014;8(6):693–702. https://doi.org/10.1586/17476348.2014.948856.
Denault A, Deschamps A, Murkin JM. A proposed algorithm for the intraoperative use of cerebral near-infrared spectroscopy. Semin Cardiothorac Vasc Anesth. 2007;11(4):274–81. https://doi.org/10.1177/1089253207311685.
Yagi T, Nagao K, Kawamorita T, Soga T, Ishii M, Chiba N, et al. Detection of ROSC in patients with cardiac arrest during chest compression using NIRS: a pilot study. Adv Exp Med Biol. 2016;876:151–7. https://doi.org/10.1007/978-1-4939-3023-4_19.
Genbrugge C, Dens J, Meex I, Boer W, Eertmans W, Sabbe M, et al. Regional cerebral oximetry during cardiopulmonary resuscitation: useful or useless? J Emerg Med. 2016;50(1):198–207. https://doi.org/10.1016/j.jemermed.2015.03.043.
Schewe JC, Thudium MO, Kappler J, Steinhagen F, Eichhorn L, Erdfelder F, et al. Monitoring of cerebral oxygen saturation during resuscitation in out-of-hospital cardiac arrest: a feasibility study in a physician staffed emergency medical system. Scand J Trauma Resusc Emerg Med. 2014;22:58. https://doi.org/10.1186/s13049-014-0058-y.
Singer AJ, Ahn A, Inigo-Santiago LA, Thode HC Jr, Henry MC, Parnia S. Cerebral oximetry levels during CPR are associated with return of spontaneous circulation following cardiac arrest: an observational study. Emerg Med J. 2015;32(5):353–6. https://doi.org/10.1136/emermed-2013-203467.
Shapiro DE. The interpretation of diagnostic tests. Stat Methods Med Res. 1999;8(2):113–34. https://doi.org/10.1177/096228029900800203.
Nishiyama K, Ito N, Orita T, Hayashida K, Arimoto H, Abe M, et al. Characteristics of regional cerebral oxygen saturation levels in patients with out-of-hospital cardiac arrest with or without return of spontaneous circulation: a prospective observational multicentre study. Resuscitation 2015;96:16–22. https://doi.org/10.1016/j.resuscitation.2015.07.013.
Perkins GD, Handley AJ, Koster RW, Castren M, Smyth MA, Olasveengen T, et al. European Resuscitation Council Guidelines for Resuscitation 2015: Sect. 2. Adult basic life support and automated external defibrillation. Resuscitation 2015;95:81–99. https://doi.org/10.1016/j.resuscitation.2015.07.015.
Koyama Y, Wada T, Lohman BD, Takamatsu Y, Matsumoto J, Fujitani S, Taira Y. A new method to detect cerebral blood flow waveform in synchrony with chest compression by near-infrared spectroscopy during CPR. Am J Emerg Med. 2013;31(10):1504–8. https://doi.org/10.1016/j.ajem.2013.07.002.
Kamarainen A, Sainio M, Olkkola KT, Huhtala H, Tenhunen J, Hoppu S. Quality controlled manual chest compressions and cerebral oxygenation during in-hospital cardiac arrest. Resuscitation 2012;83(1):138–42. https://doi.org/10.1016/j.resuscitation.2011.09.011.
Singer AJ, Nguyen RT, Ravishankar ST, Schoenfeld ER, Thode HC Jr, Henry MC, Parnia S. Cerebral oximetry versus end tidal CO2 in predicting ROSC after cardiac arrest. Am J Emerg Med. 2018;36(3):403–7. https://doi.org/10.1016/j.ajem.2017.08.046.
Chandrasekharan P, Vali P, Rawat M, Mathew B, Gugin SF, Koenigsknecht C, et al. Continuous capnography monitoring during resuscitation in a transitional large mammalian model of asphyxial cardiac arrest. Pediatr Res. 2017;81(6):898–904. https://doi.org/10.1038/pr.2017.26.
Sheak KR, Wiebe DJ, Leary M, Babaeizadeh S, Yuen TC, Zive D, et al. Quantitative relationship between end-tidal carbon dioxide and CPR quality during both in-hospital and out-of-hospital cardiac arrest. Resuscitation 2015;89:149–54. https://doi.org/10.1016/j.resuscitation.2015.01.026.
Pokorna M, Necas E, Kratochvil J, Skripsky R, Andrlik M, Franek O. A sudden increase in partial pressure end-tidal carbon dioxide (P(ET)CO(2)) at the moment of return of spontaneous circulation. J Emerg Med. 2010;38(5):614–21. https://doi.org/10.1016/j.jemermed.2009.04.064.
Asim K, Gokhan E, Ozlem B, Ozcan Y, Deniz O, Kamil K, et al. Near infrared spectrophotometry (cerebral oximetry) in predicting the return of spontaneous circulation in out-of-hospital cardiac arrest. Am J Emerg Med. 2014;32(1):14–7. https://doi.org/10.1016/j.ajem.2013.09.010.
Schnaubelt S, Sulzgruber P, Menger J, Skhirtladze-Dworschak K, Sterz F, Dworschak M. Regional cerebral oxygen saturation during cardiopulmonary resuscitation as a predictor of return of spontaneous circulation and favourable neurological outcome—a review of the current literature. Resuscitation 2018;125:39–47. https://doi.org/10.1016/j.resuscitation.2018.01.028.
Reynolds JC, Salcido D, Koller AC, Sundermann ML, Frisch A, Suffoletto BP, Menegazzi JJ. Tissue oximetry by near-infrared spectroscopy in a porcine model of out-of-hospital cardiac arrest and resuscitation. Resuscitation 2013;84(6):843–7. https://doi.org/10.1016/j.resuscitation.2012.11.031.
Putzer G, Braun P, Strapazzon G, Toferer M, Mulino M, Glodny B, et al. Monitoring of brain oxygenation during hypothermic CPR—a prospective porcine study. Resuscitation 2016;104:1–5. https://doi.org/10.1016/j.resuscitation.2016.03.027.
Acknowledgements
We thank Imran Sayed, MD, Arij Beshish, MD, Ph.D. for their diligent work in collecting data during experiments. We also thank Allison Brodbeck for surgical and administrative support of this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Eldridge received salary support for research from the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (R01 HL115061-04 and R01 HL105820) and the U.S. Department of Defense, Navy (N00024-17-C-4318 and N0463A-12-C-0004). The remaining authors have disclosed that they do not have any potential conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al-Subu, A.M., Hacker, T.A., Eickhoff, J.C. et al. Two-site regional oxygen saturation and capnography monitoring during resuscitation after cardiac arrest in a swine pediatric ventricular fibrillatory arrest model. J Clin Monit Comput 34, 63–70 (2020). https://doi.org/10.1007/s10877-019-00291-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-019-00291-2