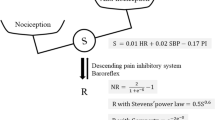
Abstract
The aim of the present study was to develop and validate an objective index for nociception level (NoL) of patients under general anesthesia, based on a combination of multiple physiological parameters. Twenty-five patients scheduled for elective surgery were enrolled. For clinical reference of NoL, the combined index of stimulus and analgesia was defined as a composite of the surgical stimulus level and a scaled effect-site concentration of opioid. The physiological parameters heart rate, heart rate variability (0.15–0.4 Hz band power), plethysmograph wave amplitude, skin conductance level, number of skin conductance fluctuations, and their time derivatives, were extracted. Two techniques to incorporate these parameters into a single index representing the NoL have been proposed: NoLlinear, based on an ordinary linear regression, and NoLnon-linear, based on a non-linear Random Forest regression. NoLlinear and NoLnon-linear significantly increased after moderate to severe noxious stimuli (Wilcoxon rank test, p < 0.01), while the individual parameters only partially responded. Receiver operating curve analysis showed that NoL index based on both techniques better discriminated noxious and non-noxious surgical events [area under curve (AUC) = 0.97] compared with individual parameters (AUC = 0.56–0.74). NoLnon-linear better ranked the level of nociception compared with NoLlinear (R = 0.88 vs. 0.77, p < 0.01). These results demonstrate the superiority of multi-parametric approach over any individual parameter in the evaluation of nociceptive response. In addition, advanced non-linear technique may have an advantage over ordinary linear regression for computing NoL index. Further research will define the usability of the NoL index as a clinical tool to assess the level of nociception during general anesthesia.





Similar content being viewed by others
References
Holte K, Kehlet H. Epidural anaesthesia and analgesia–effects on surgical stress responses and implications for postoperative nutrition. Clin Nutr. 2002;21:199–206.
Kehlet H, Jensen T, Woolf C. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–25.
Brook A, Ahrens T, Schaiff R, Prentice D, Sherman G, Shannon W, Kollef M. Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Crit Care Med. 1999;27:2609–15.
Kress J, Pohlman A, O’Connor M, Hall J. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–7.
Nevius K, Yvonne D. Proper pain management. OR Nurse. 2008;2:39–40.
Mascia M, Koch M, Medicis J. Pharmacoeconomic impact of rational use guidelines on the provision of analgesia, sedation, and neuromuscular blockade in critical care. Crit Care Med. 2000;28:2300–6.
Jänig W. Autonomic reactions in pain. Pain. 2012;153:733–936.
Benarroch E. Pain-autonomic interactions. Neurol Sci. 2006;27:130–3.
Schlereth T, Birklein F. The sympathetic nervous system and pain. Neuromol Med. 2008;10:141–7.
Loeser JD, Treede RD. The Kyoto protocol of IASP basic pain terminology. Pain. 2008;137:473–7.
Dowling J. Autonomic measures and behavioral indices of pain sensitivity. Pain. 1983;16:193–200.
Evans J. Clinical signs and autonomic responses. In: Rosen M, Lunn JN, editors. Consciousness, awareness and pain in general anaesthesia. London: Butterworth; 1987. p. 18–34.
Latson T, O’flaherty O. Effects of surgical stimulation on autonomic reflex function: assessment by changes in heart rate variability. Br J Anaesth. 1993;70:301–5.
Logier R, Jeanne M, Tavernier B, De jonckheere J (2006) Pain/analgesia evaluation using heart rate variability analysis. Engineering in Medicine and Biology Society. EMBS’06, 28th annual international conference of the IEEE.
Jeanne M, Clément C, De Jonckheere J, Logier R, Tavernier B. Variations of the analgesia nociception index during general anaesthesia for laparoscopic abdominal surgery. J Clin Monit Comput. 2012;26:289–94.
Jeanne M, Logier R, De Jonckheere J, Tavernier B. Heart rate variability during total intravenous anesthesia: effects of nociception and analgesia. Auton Neurosci. 2009;147:91–6.
Korhonen I, Yli-Hankala A. Photoplethysmography and nociception. Acta Anaesth Scand. 2009;53:975–85.
Luginbühl M, Reichlin F, Sigurdsson G, Zbinden A, Peterson-Felix S. Prediction of the haemodynamic response to tracheal intubation: comparison of laser-Doppler skin vasomotor reflex and pulse wave reflex. Br J Anaesth. 2002;89:389–97.
Murray W, Foster P. The peripheral pulse wave: information overlooked. J Clin Monit Comput. 1996;12:365–77.
Storm H, Shafiei M, Myre K, Raeder J. Palmar skin conductance compared to a developed stress score and to noxious and awakening stimuli on patients in anaesthesia. Acta Anaesth Scand. 2005;49:798–803.
Ledowski T, Bromilow J, Paech M, Storm H, Hacking R, Schug S. Monitoring of skin conductance to assess postoperative pain intensity. Br J Anaesth. 2006;97:862–5.
Treister R, Kliger M, Zuckerman G, Aryeh I, Eisenberg E. Differentiating between heat pain intensities: the combined effect of multiple autonomic parameters. Pain. 2012;153:1807–14.
Rantanen M, Yli-Hankala A, Van Gils M, Yppärilä-Wolters H, Takala P, Huiku M, Kymäläinen M, Seitsonen E, Korhonen I. Novel multiparameter approach for measurement of nociception at skin incision during general anaesthesia. Br J Anaesth. 2006;96:367–76.
Huiku M, Uutela K, Van Gils M, Korhonen I, Kymäläinen M, Meriläinen P, Paloheimo M, Rantanen M, Takala P, Viertiö-Oja H, Yli-Hankala A. Assessment of surgical stress during general anaesthesia. Br J Anaesth. 2007;98:447–55.
Mathews D, Clark L, Johansen J, Matute E, Seshagiri C. Lower composite variability index (CVI) was associated with better clinical global impression scores. Eur J Anaesth. 2009;26(Suppl 45):30–1.
Cannesson M, Rinehart J. Innovative technologies applied to anesthesia: how will they impact the way clinicians practice? J Cardiothor Vasc Anesth. 2012;26:711–20.
Breiman L. Random forests. Mach Learn. 2001;45:5–32.
Laguna P, Moody G, Mark R. Power spectral density of unevenly sampled data by least-square analysis: performance and application to heart rate signals. IEEE Trans Biomed Eng. 1998;45:698–715.
Minto C, Schnider T, Egan T, Youngs E, Lemmens H, Gambus P, Billard V, Hoke J, Moore K, Hermann DJ, Muir KT, Mandema JW, Shafer SL. Influence of age and gender on the pharmacokinetics and pharmacodynamics of remifentanil: I. Model development. Anesthesiology. 1997;86:10–23.
Shafer S, Varvel J, Aziz N, Scott JC. Pharmacokinetics of fentanyl administered by computer-controlled infusion pump. Anesthesiology. 1990;73:1091–102.
Egan T, Muir K, Hermann D, Stanski D, Shafer S. The electroencephalogram (EEG) and clinical measures of opioid potency: defining the EEG-clinical potency relationship (‘fingerprint’) with application to remifentanil. Int J Pharm Med. 2001;15:11–9.
Lemmens H, Dyck J, Shafer S, Stanski D. Pharmacokinetic-pharmacodynamic modeling in drug development: application to the investigational opioid trefentanil. Clin Pharmacol Ther. 1994;56:261–71.
Egan T, Minto C, Hermann D, Barr J, Muir K, Shafer S. Remifentanil versus alfentanil: comparative pharmacokinetics and pharmacodynamics in healthy adult male volunteers. Anesthesiology. 1996;84:821–33.
Hastie T, Tibshirani R, Friedman J. The elements of statistical learning: data mining, inference and prediction. 2nd ed. New York: Springer; 2009.
Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36.
Johnson K, Syroid N, Gupta D, Manyam S, Egan T, Huntington J, White J, Tyler D, Westenskow D. An evaluation of remifentanil propofol response surfaces for loss of responsiveness, loss of response to surrogates of painful stimuli and laryngoscopy in patients undergoing surgery. Anesth Analg. 2008;106:471–9.
Lang E, Kapila A, Shlugman D, Hoke J, Sebel P, Glass P. Reduction of isoflurane minimal alveolar concentration by remifentanil. Anesthesiology. 1996;85:721–8.
McEwan A, Smith C, Dyar O, Goodman D, Smith L, Glass P. Isoflurane minimum alveolar concentration reduction by fentanyl. Anesthesiology. 1993;78:864–9.
Sinha P, Koshy T. Monitoring devices for measuring the depth of anaesthesia—an overview. Indian J Anaesth. 2007;51:365–81.
Lu S, Zhao H, Ju K, Shin K, Lee M, Shelley K, Chon KH. Can photoplethysmography variability serve as an alternative approach to obtain heart rate variability information? J Clin Monit Comput. 2008;22:23–9.
Gil E, Orini M, Bailón R, Vergara JM, Mainardi L, Laguna P. Photoplethysmography pulse rate variability as a surrogate measurement of heart rate variability during non-stationary conditions. Physiol Meas. 2010;31:1271–90.
Matsukawa T, Kurz A, Sessler DI, et al. Propofol linearly reduces the vasoconstriction and shivering thresholds. Anesthesiology. 1995;82:1169–80.
Xiong J, Kurz A, Sessler DI, et al. Isoflurane produces marked and nonlinear decreases in the vasoconstriction and shivering thresholds. Anesthesiology. 1996;85:240–5.
Heyse B, Proost JH, Schumacher PM, Bouillon TW, Vereecke HE, Eleveld DJ, Luginbühl M, Struys MM. Sevoflurane remifentanil interaction: comparison of different response surface models. Anesthesiology. 2012;116:311–23.
Bouillon TW, Bruhn J, Radulescu L, Andresen C, Shafer TJ, Cohane C, Shafer SL. Pharmacodynamic interaction between propofol and remifentanil regarding hypnosis, tolerance of laryngoscopy, bispectral index, and electroencephalographic approximate entropy. Anesthesiology. 2004;100:1072–353.
Declaration of interest
The study was funded by Medasense Biometrics Ltd. N.B., M.K., and G.Z. are employees of Medasense Biometrics. Y.K. serves on the advisory board of Medasense Biometrics Ltd.
Author contributions
All authors jointly designed the study; R.E. and Y.K. recruited the patients, coordinated and supervised administration of anesthesia during operations; R.E., N.B., and G.Z. collected and annotated the data; N.B., M.K., and G.Z. developed NoL index and CISA score and wrote MATLAB code; N.B. and M.K. designed and performed the statistical analysis; N.B., M.K., and R.E. wrote the manuscript; G.Z. and Y.K. edited the manuscript. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ben-Israel, N., Kliger, M., Zuckerman, G. et al. Monitoring the nociception level: a multi-parameter approach. J Clin Monit Comput 27, 659–668 (2013). https://doi.org/10.1007/s10877-013-9487-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-013-9487-9