Abstract
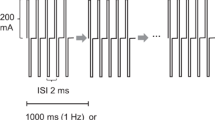
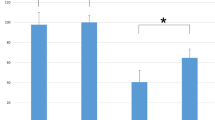
Transcranial motor evoked potentials (TcMEPs) are widely used to monitor motor function during spinal surgery. However, they are much smaller and more variable in amplitude than responses evoked by maximal peripheral nerve stimulation, suggesting that a limited number of spinal motor neurons to the target muscle are excited by transcranial stimulation. The aim of this study was to quantify the proportion of motor neurons recruited during TcMEP monitoring under general anesthesia. In twenty patients who underwent thoracic and/or lumbar spinal surgery with TcMEP monitoring, the triple stimulation technique (TST) was applied to the unilateral upper arm intraoperatively. Total intravenous anesthesia was employed. Trains of four stimuli were delivered with maximal intensity and an inter-pulse interval of 1.5 ms. TST responses were recorded from the abductor digiti minimi muscle, and the negative peak amplitude and area were measured and compared between the TST test (two collisions between transcranial and proximal and distal peripheral stimulation) and control response (two collisions between two proximal and one distal peripheral stimulation). The highest degree of superimposition of the TST test and control responses was chosen from several trials per patient. The average ratios (test:control) were 17.1 % (range 1.8–38 %) for the amplitudes and 21.6 % (range 2.9–40 %) for the areas. The activity of approximately 80 % of the motor units to the target muscle cannot be detected by TcMEP monitoring. Therefore, changes in evoked potentials must be interpreted cautiously when assessing segmental motor function with TcMEP monitoring.



Similar content being viewed by others
References
Jellinek D, Jewkes D, Symon L. Noninvasive intraoperative monitoring of motor evoked potentials under propofol anesthesia: effect of spinal surgery on the amplitude and latency of motor evoked potentials. Neurosurgery. 1991;29:551–7.
Keller BP, Haghighi SS, Oro JJ, Eggerrs GWN. The effect of propofol anesthesia on transcortical electric evoked potentials in the rat. Neurosurgery. 1992;30:557–60.
Taylor BA, Fennelly ME, Taylor A, Farrell J. Temporal summation—the key to motor evoked potential spinal cord monitoring in humans. J Neurol Neurosurg Psychiat. 1993;56:104–6.
Jones SJ, Harrison R, Koh KF, Mendoza N, Crockard HA. Motor evoked potential monitoring during spinal surgery: response of distal limb muscle to transcranial cortical stimulation with pulse train. Electroenceph Clin Neurophysiol. 1996;100:375–83.
Pechstein U, Cedzich C, Nadstawek J, Schramm J. Transcranial high-frequency repetitive electrical stimulation for recording myogenic motor evoked potentials with the patient under general anesthesia. Neurosurgery. 1996;39:335–44.
Woodforth IJ, Hicks RG, Crawford MR, Stephen JP, Burke DJ. Variability of motor-evoked potentials recording during nitrous oxide anesthesia from the tibialis anterior muscle after transcranial electrical stimulation. Anesth Analg. 1996;82:744–9.
Magistris MR, Rosler KM, Truffert A, Myers JP. Transcranial stimulation excites virtually all motor neurons supplying the target muscle. Brain. 1998;121:437–50.
Matsuda H, Shimazu A. Intraoperative spinal cord monitoring using electric responses to stimulation of caudal spinal cord or motor cortex. In: Desmedt JE, editor. Neuromonitoring in surgery. Amsterdam: Elsevier; 1989. p. 175–90.
Merton PA, Morton HB. Stimulation of the cerebral cortex in the intact human subject. Nature. 1980;285:227.
Merton PA, Morton HB. Electrical stimulation of human motor and visual cortex through the scalp. J Physiol. 1980;305:9P–10P.
Zentner J. Noninvasive motor evoked potential monitoring during neurosurgical operations on the spinal cord. Neurosurgery. 1989;24:709–12.
Calancie B, Klose KL, Baier S, Green BA. Isoflurane-induced attenuation of motor evoked potentials caused by electrical motor cortex stimulation during surgery. J Neurosurg. 1991;74:897–904.
Deletis V, Sala F. Intraoperative neurophysiological monitoring of the spinal cord during spinal cord and spine surgery: a review focus on the corticospinal tracts. Clin Neurophysiol. 2008;119:248–64.
Leppanen RE. Faces of spine care. From the electrodiagnostic lab: where transcranial stimulation, H-reflexes and F-responses monitor cord function intraoperatively. Spine J. 2004;4:601–3.
Leppanen RE. Intraoperative applications of the H-reflex and F-response: a tutorial. J Clin Monit Comput. 2006;20:267–304.
Sloan TB, Janik D, Jameson L. Multimodality monitoring of the central nervous system using motor-evoked potentials. Curr Opin Anesthesiol. 2008;21:560–4.
Malhotra NR, Shaffrey CI. Intraoperative electrophysiological monitoring in spine surgery. Spine (Phila Pa 1976). 2010;35:2167–79.
Taniguchi M, Cedzich C, Schramm J. Modification of cortical stimulation for motor evoked potentials under general anesthesia: technical description. Neurosurgery. 1993;32:219–26.
Iwasaki H, Tamaki T, Yoshida M, Ando M, Yamada H, Tsutsui S, Takami M. Efficacy and limitations of current methods of intraoperative spinal cord monitoring. J Orthop Sci. 2003;8:635–42.
Journee HL, Polak HE, de Kleuver M, Langeloo DD, Postma AA. Improved neuromonitoring during spinal surgery using double-train transcranial electrical stimulation. Med Biol Eng Comput. 2004;42:110–3.
Kakimoto M, Kawaguchi M, Yamamoto Y, Inoue S, Horiuchi T, Nakase H, Sakaki T, Furuya H. Tetanic stimulation of the peripheral nerve before transcranial electrical stimulation can enlarge amplitudes of myogenic motor evoked potentials during general anesthesia with neuromuscular blockade. Anesthesiology. 2005;102:733–8.
Frei FJ, Ryhult SE, Duitmann E, Hasler CC, Luetschg J, Erb TO. Intraoperative monitoring of motor evoked potentials in children undergoing spinal surgery. Spine (Phila Pa 1976). 2007;32:911–7.
Conflict of interest
No funds were received in support of this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Ethical standard
The experiments comply with the current laws of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsutsui, S., Yamada, H., Hashizume, H. et al. Quantification of the proportion of motor neurons recruited by transcranial electrical stimulation during intraoperative motor evoked potential monitoring. J Clin Monit Comput 27, 633–637 (2013). https://doi.org/10.1007/s10877-013-9480-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-013-9480-3