Abstract
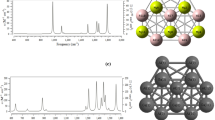
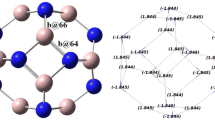
The adsorption analysis of some organic inhibitors consisting of Pyridine, 2-Methylpyridine, 3-Methylpyridine, 4-Methylpyridine and 2, 4-Dimethylpyridine onto aluminum (111) metal surface based on optimized coordination of binding on the Al (111) metal surface has been accomplished. In this research, the ONIOM approach has been performed with a three-layered level of high level of DFT method using 6–31 + G* and LANL2DZ basis sets by the physico-chemical software of Gaussian 09, a medium semi-active part that includes important electronic contributions, and a low level part that has been handled using MM2 force field approaches. The physico-chemical properties of adsorption -surface complexes are one of the principal parameters for determining and choosing the adsorption. The characteristic of the metal (111)-lattice in solutions of hydrochloric acid and nitric acid by some heterocyclic compounds including Pyridine, 2-Methylpyridine, 3-Methylpyridine, 4-Methylpyridine and 2, 4-Dimethylpyridine has been estimated through NMR and IR results. Nitrogen atom in pyridine cycle with the most influence on the NMR shielding of isotropic and anisotropic tensors has leaded us toward the active site for adsorption onto Al (111) surface. Moreover, the IR spectrum for each of these heterocyclic compounds consisting of Pyridine, 2-Methylpyridine, 3-Methylpyridine, 4-Methylpyridine and 2, 4-Dimethylpyridine has been indicted in the frequency range between 500 cm−1 and 3250 cm−1 by the strongest peaks about 550 cm−1, 1500 cm−1, and 3250 cm−1. In this work, the co-adsorption of Cl− / NO3− anions with H+ads cation onto aluminum-surface through the optimized adsorption energy for these compounds on a top site of metal (111) has been accomplished. Our calculations have illustrated that the adsorption stability energy of pyridine and its derivatives depends on the structure, the adsorption site and the acidic media.










Similar content being viewed by others
References
G. Tarantella (1991). Corrosion 47, 410.
G. Schmitt (1984). Corros. J. 19, 165.
J. O. M. Bockris and B. Yang (1991). J. Electrochem. Soc. 138, 2237.
F. B. Growcock and V. R. Lopp (1988). Corros. Sci. 28, 397.
M. Bartos and N. Hackerman (1992). J. Electrochem. Soc. 139 (3), 429.
F. Zucchi, G. Trabanelli, and G. Brunoro (1992). Corros. Sci. 33 (1), 135.
S. L. Graaese (1988). Corrosion. 44, 222.
A. B. Tadros and B. A. Abdenaby (1988). J. Electroanal. Chem. 246, 433.
B. Mernari, H. Elattari, M. Traisnel, F. Bentiss, and M. Lagreence (1998). Corros. Sci. 40, 391.
F. Bentiss, M. Lagreence, and M. Traisnel (1999). Corros. Sci. 41, 789.
F. Bentiss, M. Traisnel, and M. Lagreence (2000). Corros. Sci. 42, 127.
D. Xue Ging (1990). J. Appl. Surf. Sci. 40, 327.
D. Chadwick and T. Hashemi (1978). Corros. Sci. 20, 88.
J. J. Xue Gadding (1991). J. Phys. Chem. 95, 7380.
Khaleghian, M.; Zahmatkesh, M.; Mollaamin, F.; Monajjemi, M. (2011) Investigation of Solvent Effects on Armchair Single-Walled Carbon Nanotubes: A QM/MD Study. Fullerenes Nanotubes and Carbon Nanostructures 19
L. Ming-Dao, B. Gang, K. Fu-Gui, Y. Lu-An, and Y. Xiao-Ci (1996). Acta Chemica. 12 (9), 859.
L. Ming-Dao, Y. Lu-An, W. Qing-Yu, Y. Xiao-Ci, Y. Xiao-Dong, and Z. Jin-Yun (1996). J. Chinese Soc. Corrosion Protec. 16 (3), 195.
C. Wei (2019). Electrochemical deposition of aluminum. Technol. Innov. Appl. 18, 80–81.
H. M. Yang, Z. X. Qiu, and G. Zhang, Low Temperature Aluminum Electrolysis (Northeast University Press, Shenyang, China, 2009).
H. M. Lu and Z. X. Qiu (1997). Research progress of low temperature aluminum electrolysis. Light Metals 4, 24.
M. Monajjemi, M. T. Baie, and F. Mollaamin (2010). Interaction between threonine and cadmium cation in [Cd(Thr) n ]2+ (n = 1–3) complexes: density functional calculations. Russian Chemical Bulletin 59, 886–889.
F. Mollaamin and M. Monajjemi (2015). Harmonic linear combination and normal mode analysis of semiconductor nanotubes vibrations. J. Comput. Theor. Nanosci. 12, 1030–1039.
P. Wasserscheid and T. Welton, Ionic Liquids in Synthesis; John Wiley & Sons: Hoboken (NJ, USA, 2008).
M. Zhang, V. Kamavarum, and R. G. Reddy (2003). New electrolytes for aluminum production. Ionic Liquids. JOM 55, 54.
G. C. Tian, J. Li, and Y. X. Hua (2009). Application of ionic liquids in metallurgy of nonferrous metals. Chin. J. Process Eng. 9, 200.
G. C. Tian, J. Li, and Y. X. Hua (2010). Application of ionic liquids in hydrometallurgy of nonferrous metals. Trans. Nonferrous Met. Soc. China 20, 513.
M. Monajjemi, S. Afsharnezhad, M. R. Jaafari, S. Mirdamadi, F. Mollaamin, and H. Monajemi (2008). Investigation of energy and NMR isotropic shift on the internal rotation Barrier of Θ4 dihedral angle of the DLPC: A GIAO study. Chemistry 17, 55–69.
G. C. Tian (2019). Ionic liquids as green electrolytes for Aluminum and Aluminum-alloy production. Mater. Res. Found. 54, 249.
X. W. Zhong, T. Xiong, J. Lu, and Z. N. Shi (2014). Advances of electro-deposition and aluminum refining of aluminum and aluminum alloy in ionic liquid electrolytes system. Nonferrous Met. Sci. Eng. 5, 44.
Y. Zheng, Q. Wang, Y. J. Zheng, and H. C. Lv (2015). Advances in research and application of aluminum electrolysis in ionic liquid systems. Chin. J. Process Eng. 15, 713.
V. Fleury, J. H. Kaufman, and D. B. Hibbert (1994). Mechanism of a morphology transition in ramified electrochemical growth. Nature 367, 435.
G. Yue, X. Lu, Y. Zhu, X. Zhang, and S. Zhang (2009). Surface morphology, crystal structure and orientation of aluminum coatings electrodeposited on mild steel in ionic liquid. Chem. Eng. J. 147, 79.
A. P. Abbott, F. Qiu, H. M. Abood, M. R. Ali, and K. S. Ryder (1862). Double layer, diluent and anode effects upon the electrodeposition of aluminum from chloroaluminate based ionic liquids. Phys. Chem. Chem. Phys. 2010, 12.
M. Monajjemi, L. Mahdavian, F. Mollaamin, and M. Khaleghian (2009). Interaction of Na, Mg, Al, Si with carbon nanotube (CNT): NMR and IR study. Russian J. Inorg. Chem. 54, 1465–1473.
M. Ueda, S. Hariyama, and T. Ohtsuka (2012). Al electroplating on the AZ121 Mg alloy in an EMIC-AlCl3 ionic liquid containing ethylene glycol. J. Solid State Electrochem. 16, 3423.
Q. Zhang, Q. Wang, S. Zhang, and X. Lu (2014). Effect of nicotinamide on electrodeposition of Al from aluminum chloride (AlCl3)-1-butyl-3-methylimidazolium chloride ([BMIM]Cl) ionic liquids. J. Solid State Electrochem. 18, 257.
M. Monajjemi, M. Khaleghian, N. Tadayonpour, and F. Mollaamin (2010). The effect of different solvents and temperatures on stability of single-Walled carbon nanotube: A Qm/Md study. Int. J. Nanosci. 09, 517–529.
B. Ghalandari, M. Monajjemi, and F. Mollaamin (2011). Theoretical investigation of carbon nanotube binding to DNA in view of drug delivery. J. Comput. Theor. Nanosci. 8, 1212–1219.
F. Mollaamin, M. Monajjemi, S. Salemi, and M. T. Baei (2011). A dielectric effect on normal mode analysis and symmetry of BNNT Nanotube. Fullerenes Nanotubes and Carbon Nanostruc. 19, 182–196.
N. Hackerman and A. C. Makrides (1954). Ind. Engng. Chem. 46, 523.
K. Aramaki, N. Hackerman, and J. Eleclrochem (1968). Soc 115, 1007.
A.I. Altsybeeva, S.Z. Levin and A.P. Dorokhov (1970) European Symposium on Corrosion Inhibitors, Ferrera 501
J. V. Barth, H. Brune, G. Ertl, and R. J. Behm (1990). Scanning tunneling microscopy observations on the reconstructed Au(111) surface: Atomic structure, long-range superstructure, rotational domains, and surface defects. Phys. Rev. B. 42, 9307–9318.
D. Esken, S. Turner, O. I. Lebedev, G. van Tendeloo, and R. A. Fischer (2010). Au@ ZIFs: stabilization and encapsulation of cavity-size matching gold clusters inside functionalized zeolite imidazolate frameworks. ZIFs. Chem. Mater. 22, 6393–6401.
F. Mollaamin, A. Ilkhani, N. Sakhaei, B. Bonsakhteh, A. Faridchehr, S. Tohidi, and M. Monajjemi (2015). Thermodynamic and solvent effect on dynamic structures of nano bilayer-cell membrane: hydrogen bonding study. J. Comput. Theoretical Nanosci. 12, 3148–3154.
B. Liu and B. Smit (2010). Molecular simulation studies of separation of CO2/N2, CO2/CH4, and CH4/N2 by ZIFs. J. Phys. Chem. C 114, 8515–8522.
S. Keskin (2010). Atomistic simulations for adsorption, diffusion, and separation of gas mixtures in zeolite imidazolate frameworks. J. Phys. Chem. C 115, 800–807.
U. P. N. Tran, K. K. A. Le, and N. T. S. Phan (2011). Expanding applications of metal- organic frameworks: zeolite imidazolate framework ZIF-8 as an efficient heterogeneous catalyst for the knoevenagel reaction. ACS Catal. 1, 120–127.
J. VandeVondele, M. Krack, F. Mohamed, M. Parrinello, T. Chassaing, and J. Hutter (2005). Quickstep: Fast and accurate density functional calculations using a mixed Gaussian and plane waves approach. Comp. Phys. Comm. 2, 103–128.
A. Phan, C. J. Doonan, F. J. Uribe-Romo, C. B. Knobler, and O’keeffe, M., Yaghi, O. M. (2010). Synthesis, structure, and carbon dioxide capture properties of zeolitic imidazolate frameworks. Acc. Chem. Res. 43, 58–67.
P. Hohenberg and W. Kohn (1964). Inhomogeneous electron gas. Phys. Rev. B 136, B864–B871.
W. Kohn and L. J. Sham (1965). Self-consistent equations including exchange and correlation effects. Phys. Rev. 140, A1133–A1138.
G. Lippert, J. Hutter, and M. Parrinello (1997). A hybrid Gaussian and plane wave density functional scheme. Mol. Phys. 92, 477–487.
C. Hartwigsen, S. Goedecker, and J. Hutter (1998). Relativistic separable dual-space Gaussian pseudopotentials from H to Rn. Phys. Rev. B 58, 3641–3662.
J. P. Perdew, K. Burke, and M. Ernzerhof (1996). Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 77, 3865–3868.
J. VandeVondele and J. Hutter (2007). Gaussian basis sets for accurate calculations on molecular systems in gas and condensed phases. J. Chem. Phys. 127, 114105–114109.
M. Monajjemi, M. Noei, and F. Mollaamin (2010). Design of fMet-tRNA and calculation of its bonding properties by quantum mechanics. Nucleosides, Nucleotides & Nucleic Acids 29, 676–683.
M. Mavrikakis, P. Stoltze, and J. K. Nørskov (2000). Making gold less noble. Catal. Lett. 64 (101–106), 59.
A. Dal Corso (2013). Ab initio phonon dispersions of transition and noble metals: effects of the exchange and correlation functional. J. Phys.; Condens Matter 25, 145401–145410.
L. J. Whitman, J. A. Stroscio, R. A. Dragoset, and R. J. Celotta (1991). Geometric and electronic properties of Cs structures on III-V (110) surfaces: From 1D and 2D insulators to 3D metals. Phys. Rev. Lett. 66, 1338–1341.
M. A. A. Zadeh, H. Lari, L. Kharghanian, E. Balali, R. Khadivi, H. Yahyaei, F. Mollaamin, and M. Monajjemi (2015). Density functional theory study and anti-cancer properties of shyshaq plant. in view point of nano biotechnology. J. Comput. Theoret. Nanosci. 12, 4358–4367.
H. Yildirim, T. Greber, and A. Kara (2013). Trends in adsorption characteristics of benzene on transition metal surfaces: role of surface chemistry and van der waals interactions. J. Phys. Chem. C 117, 20572–20583.
A. Tahan, F. Mollaamin, and M. Monajjemi (2009). Thermochemistry and NBO analysis of peptide bond: Investigation of basis sets and binding energy. Russian J. Phys. Chem. A 83, 587–597.
M. Hoefling, F. Iori, S. Corni, and K. E. Gottschalk (2010). The conformations of amino acids on a Gold(111) surface. Chem. Phys. Chem. 11, 1763–1767.
K. Bakhshi, F. Mollaamin, and M. Monajjemi (2011). Exchange and correlation effect of hydrogen chemisorption on nano V(100) Surface: A DFT study by generalized gradient approximation (GGA). J. Comput. Theoret. Nanosci. 8, 763–768. https://doi.org/10.1166/jctn.2011.1750.
B. Cordero, V. Gómez, A. E. Platero-Prats, M. Revés, J. Echeverría, E. Cremades, F. Barragán, and S. Alvarez (2008). Covalent radii revisited. Dalton Trans. 21, 2832–2838.
A. van der Bondii (1964). Waals Volumes and Radii. Phys. Chem. 68, 441–451.
H. Valencia, M. Kohyama, S. Tanaka, and H. Matsumoto (2008). Ab initio study of EMIM-BF4 molecule adsorption on Li surfaces as a model for ionic liquid/Li interfaces in Li-ion batteries. Phys. Rev. B 78, 205402.
H. Valencia, M. Kohyama, S. Tanaka, and H. Matsumoto (2009). Ab initio study of EMIM-BF4 crystal interaction with a Li (100) surface as a model for ionic liquid/Li interfaces in Li-ion batteries. J. Chem. Phys. 131, 244705.
J. Clarke-Hannaford, M. Breedon, A. S. Best, and M. J. Spencer (2019). The interaction of ethylammoniumtetrafluoroborate [EtNH3+][BF4−] ionic liquid on the Li (001) surface, towards understanding early SEI formation on Li metal. Phys. Chem. Chem. Phys. 21, 10028–10037.
Q. Q. Zhang, Study on Electrodeposition of Aluminum and Aluminum Alloy in Ionic Liquid (University of Chinese Academy of Sciences, Beijing, China, 2014).
E. L. Kolsbjerg, M. N. Groves, and B. Hammer (2016). Pyridine adsorption and diffusion on Pt(111) investigated with density functional theory. J. Chem. Phys. 144, 164112.
S. Shimizu, N. Watanabe, T. Kataoka, T. Shoji, N. Abe, S. Morishita, and H. Ichimura (2000). Pyridine and pyridine derivatives. Ullmann’s Encyclopedia of Industrial Chemistry Weinheim: Wiley-VCH. https://doi.org/10.1002/14356007.a22_399.
E. J. O’Loughlin, S. J. Traina, and G. K. Sims (2000). Effects of sorption on the biodegradation of 2-methylpyridine in aqueous suspensions of reference clay minerals. Environ. Toxicol. Chem. 19 (9), 2168–2174. https://doi.org/10.1002/etc.5620190904.
Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; et al. Gaussian 09, Revision B.01. 2010. Gaussian Inc., Wallingford
A. Fry, K. Kwon, S. D. Komarneni, J. T. Kubicki, and K. Mueller (2006). Solid-state NMR and computational chemistry study of mononucleotides adsorbed to alumina. Langmuir 22, 9281–9286.
M. Svensson, S. Humbel, R. D. J. Froese, T. Matsubara, S. Sieber, and K. Morokuma (1996). ONIOM: A Multilayered Integrated MO + MM method for geometry optimizations and single point energy predictions. A Test for diels−alder reactions and Pt(P(t-Bu)3)2 + H2 oxidative addition. J. Phys. Chem. 100 (50), 19357–19363. https://doi.org/10.1021/jp962071j.
Felix Brandt and Christoph R. Jacob (2022). Systematic QM Region construction in QM/MM calculations based on uncertainty quantification. J. Chem. Theory Comput. 18 (4), 2584–2596. https://doi.org/10.1021/acs.jctc.1c01093.
A. Bilić, J. R. Reimers, and Hush.N.S. (2002). Adsorption of pyridine on the gold (111) surface: implications for “Alligator Clips” for molecular wires. J. Phys. Chem. B 106 (26), 6740–6747. https://doi.org/10.1021/jp020590i.
Walter Malone and Abdelkader Kara (2020). A coverage dependent study of the adsorption of pyridine on the (111) coinage metal surfaces. Surface Science 693, 121525. https://doi.org/10.1016/j.susc.2019.121525.
M. Monajjemi, F. Mollaamin, M. R. Gholami, H. Yoosbashizadeh, S. K. Sadrnezhad, and H. Passdar (2003). Quantum chemical parameters of some organic corrosion inhibitors, Pyridine, 2-Picoline 4-Picoline and 2, 4- Lutidine, adsorption at aluminum surface in hydrocholoric and nitric acids and comparison between two acidic media. Main Group Met. Chem. 26, 349–362.
Elodie Dumont, Charlotte De Bleye, Merzouk Haouchine, Laureen Coïc, Pierre-Yves. Sacré, Philippe Hubert, and Eric Ziemons (2020). Effect of the functionalisation agent on the surface-enhanced Raman scattering (SERS) spectrum: Case study of pyridine derivatives. Spectrochimica Acta Part A: Mol. Biomol. Spectroscopy 233, 118180. https://doi.org/10.1016/j.saa.2020.118180.
Doreen Mollenhauer, Nicola Gaston, Elena Voloshina, and Beate Paulus (2013). Interaction of pyridine derivatives with a gold (111) Surface as a model for adsorption to large nanoparticles. J. Phys. Chem. C 117 (9), 4470–4479. https://doi.org/10.1021/jp309625h.
Cristina Isvoranu, Bin Wang, Evren Ataman, Karina Schulte, Jan Knudsen, Jesper N. Andersen, Marie-Laure. Bocquet, and Joachim Schnadt (2011). Pyridine adsorption on single-layer iron phthalocyanine on Au(111). The J. Phys. Chem. C 115 (41), 20201–20208. https://doi.org/10.1021/jp204460g.
A. D. Becke (1993). Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652.
C. Lee, W. Yang, and R. G. Parr (1988). Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789.
K. Kim and K. D. Jordan (1994). Comparison of density functional and MP2 calculations on the water monomer and dimer. J. Phys. Chem. 98 (40), 10089–10094. https://doi.org/10.1021/j100091a024.
P. J. Stephens, F. J. Devlin, C. F. Chabalowski, and M. J. Frisch (1994). Ab Initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 98 (45), 11623–11627. https://doi.org/10.1021/j100096a001.
C. J. Cramer (2004) Essentials of computational chemistry: theories and models, 2nd Edition | Wiley". Wiley.com. Retrieved 2021–06–24.
S. H. Vosko, L. Wilk, and M. Nusair (1980). Accurate spin-dependent electron liquid correlation energies for local spin density calculations: a critical analysis. Can. J. Phys. 58 (8), 1200–1211. https://doi.org/10.1139/p80-159.
Y. Xiao-Ci, Z. Hong, L. Ming-Dao, and R. Hong-Xuan (2000). Lu-An, Y. 42, 645–653.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mollaamin, F., Shahriari, S., Monajjemi, M. et al. Nanocluster of Aluminum Lattice via Organic Inhibitors Coating: A Study of Freundlich Adsorption. J Clust Sci 34, 1547–1562 (2023). https://doi.org/10.1007/s10876-022-02335-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-022-02335-1