Abstract
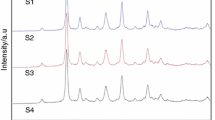
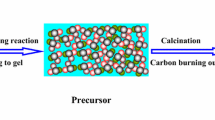
MgO–Y2O3 composite nanopowder was synthesized from Pechini method. Several experiments were conducted to examine the effects of the molar ratio of citric acid (CA) to transition metals (TM) calcination temperature and the pH value on the morphology, phases, and specific surface area of the samples. As-synthesized MgO–Y2O3 composite nanopowder were characterized by X-ray diffraction, field-emission scanning electron microscope (FESEM), transmission electron microscope, BET, thermal gravimetric–differential thermal analysis and Fourier transform infrared analysis. FESEM analysis revealed that the sample with the pH value of 1 and CA:TM = 10:1, had good homogeneity, and a spherical morphology with the particle size of 19.5 nm, while by increasing the pH up to 7 and 12, the particle size increased to 28.5 and 30.9 nm respectively. By increasing the mole ratio of CA to TM, the particle size and the specific surface area of MgO–Y2O3 composite nanopowder has reduced and by increasing the pH from 1 to 7 and 12, the particle size and the specific surface area of MgO–Y2O3 composite nanopowder has increased.













Similar content being viewed by others
References
B. H. Kear, R. Sadangi, V. Shukla, T. Stefanik, and R. Gentilman. Submicron-grained transparent yttria composites. In Proceedings SPIE 5786, Window and Dome Technologies and Materials IX, p. 227 (2005).
M. Ahsanzadeh-Vadeqani, R. S. Razavi, M. Barekat, G. H. Borhani, and A. K. Mishra (2016). Preparation of yttria nanopowders for use in transparent ceramics by dry ball-milling technique. J. Eur. Ceram. Soc. doi:10.1016/j.jeurceramsoc.2016.12.006.
R. Marder, R. Chaim, G. Chevallier, and C. Estournès (2011). Effect of 1 wt% LiF additive on the densification of nanocrystalline Y2O3 ceramics by spark plasma sintering. J. Eur. Ceram. Soc. 31, 1057–1066.
R. Marder, R. Chaim, G. Chevallier, and C. Estournes (2011). Densification and polymorphic transition of multiphase Y2O3 nanoparticles during spark plasma sintering. Mater. Sci. Eng. A 528, 7200–7206.
X. Gang, Y. Shanjiang, Y. Xujie, L. Lude, and W. Xin (1998). Preparation and characterization of nanocrystals Y2O3. J. Funct. Mater. 29, 92–95.
L. Jin, G. Zhou, S. Shimai, J. Zhang, and S. Wang (2010). ZrO2-doped Y2O3 transparent ceramics via slip casting and vacuum sintering. J. Eur. Ceram. Soc. 30, 2139–2143.
D. C. Harris (1998). Durable 3–5 μm transmitting infrared window materials. Infrared Phys. Technol. 39, 185–201.
T. Stefanik, R. Gentilman, and P. Hogan. Nanocomposite optical ceramics for infrared. In Proceedings of SPIE 6545, Window and Dome Technologies and Materials X, p. 65450A (2007).
Y. Huang, D. Jiang, J. Zhang, Q. Lin, and Z. Huang (2010). Sintering of transparent yttria ceramics in oxygen atmosphere. J. Am. Ceram. Soc. 93, 2964–2967.
Q. Zhu, A. R. Oganov, and A. O. Lyakhov (2013). Novel stable compounds in the MgO system under high pressure. Phys. Chem. Chem. Phys. 15, 7696–7700.
P. A. Tellex and J. R. Waldron (1955). Reflectance of magnesium oxide. JOSA 45, 19.
S. F. Wang, J. Zhang, D. W. Luo, F. Gu, D. Y. Tang, Z. L. Dong, G. E. B. Tan, W. X. Que, T. S. Zhang, S. Li, et al. (2013). Transparent ceramics: processing, materials and applications. Prog. Solid State Chem. 41, 20–54.
M. Ghaderi, R. S. Razavi, M. R. Loghman-Estarki, and S. Ghorbani (2016). Spark plasma sintering of transparent Y2O3 ceramic using hydrothermal synthesized nanopowders. Ceram. Int. 42, 14403–14410.
M. R. Loghman-Estarki, F. Davar, S. Ghorbani, M. Zendehdel, and M. H. Taherian (2016). Synthesis and characterization of aluminum oxy nitride (AlON) from the nanosized gel precursor. Ceram. Int. 42, 16861–16866.
R. S. Razavi, M. Ahsanzadeh-Vadeqani, M. Barekat, M. Naderi, S. H. Hashemi, and A. K. Mishra (2016). Effect of sintering temperature on microstructural and optical properties of transparent yttria ceramics fabricated by spark plasma sintering. Ceram. Int. 42, 7819–7823.
M. Hajizadeh-Oghaz, R. S. Razavi, and A. Ghasemi (2015). Synthesis and characterization of ceria–yttria co-stabilized zirconia (CYSZ) nanoparticles by sol–gel process for thermal barrier coatings (TBCs) applications. J. Sol-Gel Sci. Technol. 74, 603–612.
T.-T. Fang and J.-D. Tsay (2001). Effect of pH on the chemistry of the barium titanium citrate gel and its thermal decomposition behavior. J. Am. Ceram. Soc. 84, 2475–2478.
H.-F. Yu and K.-C. Huang (2003). Effects of pH and citric acid contents on characteristics of ester-derived BaFe 12 O 19 powder. J. Magn. Magn. Mater. 260, 455–461.
B. D. Cullity and J. W. Weymouth (1957). Elements of X-ray diffraction. Am. J. Phys. 25, 394–395.
M. Pudukudy and Z. Yaakob (2014). Catalytic aspects of ceria–zirconia solid solution: Part-I an update in the synthesis, properties and chemical reactions of ceria zirconia solid solution. Pharma Chem. 6, 188.
S. Sakka. Handbook of Sol-Gel Science and Technology. Sol-Gel Processing, vol. 1 (Springer, Berlin, 2005).
M. R. Loghman-Estarki, M. Hajizadeh-Oghaz, H. Edris, and R. S. Razavi (2013). Comparative studies on synthesis of nanocrystalline Sc2O3–Y2O3 doped zirconia (SYDZ) and YSZ solid solution via modified and classic Pechini method. CrystEngComm. 15, 5898–5909.
M. H. Oghaz, R. S. Razavi, M. R. Loghman-Estark, and R. Ghasemi (2012). Optimization of morphology and particle size of modified sol gel synthesized YSZ nanopowder using Taguchi method. J. Nano Res. 21, 65–70.
M. Hajizadeh-Oghaz, R. S. Razavi, and M. Khajelakzay (2015). Optimizing sol–gel synthesis of magnesia-stabilized zirconia (MSZ) nanoparticles using Taguchi robust design for thermal barrier coatings (TBCs) applications. J. Sol-Gel Sci. Technol. 73, 227–241.
G. Di Girolamo, C. Blasi, M. Schioppa, and L. Tapfer (2010). Structure and thermal properties of heat treated plasma sprayed ceria–yttria co-stabilized zirconia coatings. Ceram. Int. 36, 961–968.
L. L. Beecroft and C. K. Ober (1997). Nanocomposite materials for optical applications. Chem. Mater. 9, 1302–1317.
S. Xu, J. Li, H. Kou, Y. Shi, Y. Pan, and J. Guo (2015). Spark plasma sintering of Y2O3–MgO composite nanopowder synthesized by the esterification sol–gel route. Ceram. Int. 41, 3312–3317.
S. Shirinparvar, R. S. Razavi, F. Davar, M. R. Loghman-Estarki, M. Hajizadeh-Oghaz, and S. Ghorbani (2016). Synthesis, characterization and optical properties of Zr+ 4/La+ 3/Nd+ 3 tri-doped yttria nanopowder by sol–gel combustion method. Ceram. Int. 42, 10551–10558.
M. Ahsanzadeh-Vadeqani, and R. S. Razavi (2016). Spark plasma sintering of zirconia-doped yttria ceramic and evaluation of the microstructure and optical properties. Ceram. Int. 42, 18931–18936.
S. G. Cho, P. F. Johnson, and R. A. Condrate Sr. (1990). Thermal decomposition of (Sr, Ti) organic precursors during the Pechini process. J. Mater. Sci. 25, 4738–4744.
S. Wang, C. An, Y. Zhang, Z. Zhang, and Y. Qian (2006). Ethanothermal reduction to MoO2 microspheres via modified Pechini method. J. Cryst. Growth 293, 209–215.
C.-H. Chen, J. K. M. Garofano, C. K. Muoto, A. L. Mercado, S. L. Suib, M. Aindow, M. Gell, and E. H. Jordan (2011). A foaming esterification sol–gel route for the synthesis of magnesia–yttria nanocomposites. J. Am. Ceram. Soc. 94, 367–371.
S. Xu, J. Li, C. Li, Y. Pan, and J. Guo (2015). Hot pressing of infrared-transparent Y2O3–MgO nanocomposites using sol–gel combustion synthesized powders. J. Am. Ceram. Soc. 98, 1019–1026.
S. Ghorbani, R. S. Razavi, M. R. Loghman-Estarki, and A. Alhaji (2016). Synthesis of MgO–Y2O3 composite nanopowder with a high specific surface area by the Pechini method. Ceram. Int. 43, 345–354.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghorbani, S., Razavi, R.S., Loghman-Estarki, M.R. et al. Development of MgO–Y2O3 Composite Nanopowder by Pechini Sol–Gel Method: Effect of Synthesis Parameters on Morphology, Particle Size, and Phase Distribution. J Clust Sci 28, 1523–1539 (2017). https://doi.org/10.1007/s10876-017-1162-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-017-1162-8