Abstract
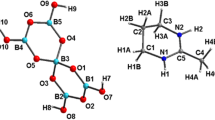
Two novel borates [(CH3)3NH][B5O6(OH)4] (I) and Na2[H2TMED][B7O9(OH)5]2 (II) have been synthesized under solvothermal conditions, and characterized by elemental analyses, FT-IR spectroscopy, and single crystal X-ray diffraction. Crystal data for I: monoclinic, P21/c, a = 9.3693(11) Å, b = 14.0375(17) Å, c = 10.0495(9) Å, β = 91.815(9)°, Z = 4. Crystal data for II: monoclinic, P21/c, a = 11.6329(2) Å, b = 11.9246(3) Å, c = 10.2528(2) Å, β = 100.178(2)°, Z = 4. Their crystal structures both have 3D supramolecular framework with large channels constructed by O–H···O hydrogen-bonding among the polyanions of [B5O6(OH)4]− or [B7O9(OH)5]2− clusters. The templating organic amines cations in I and II are both located in the channels of 3D supramolecular frameworks, respectively, and interact with the polyborate frameworks both electrostatically and via hydrogen bonds of N–H···O. Na2[H2TMED][B7O9(OH)5]2 is the first example of heptaborate co-templated by alkali metal and organic base, which is also rare in borates. The photoluminescence property of the synthetic sample of Na2[H2TMED][B7O9(OH)5]2 in the solid state at room temperature was also investigated by fluorescence spectrophotometer.






Similar content being viewed by others
References
J. D. Grice, P. C. Burns, and F. C. Hawthorne (1999). Can. Mineral. 37, 731.
D. M. Schubert (2003). Struct. Bonding. 105, 1.
P. Becker (1998). Adv. Mater. 10, 979.
A. S. Batsanov, O. V. Petrova, Yu T Struchkov, V. M. Akimov, A. K. Molodkin, and V. G. Skvortsov (1986). Zhur. Neorg. Khim. 31, 1120.
D. M. Schubert, M. Z. Visi, and C. B. Knobler (2000). Inorg. Chem. 39, 2250.
G. M. Wang, Y. Q. Sun, and G. Y. Yang (2004). J. Solid State Chem. 177, 4648.
Z. H. Liu, L. Q. Li, and W. J. Zhang (2006). Inorg. Chem. 45, 1430.
S. H. Yang, G. B. Li, S. J. Tian, F. H. Liao, M. Xiong, and J. H. Lin (2007). J. Solid State Chem. 80, 2225.
C. Y. Pan, G. M. Wang, S. T. Zheng, and G. Y. Yang (2007). Z. Anorg. Allg. Chem. 633, 336.
H. X. Liu, Y. X. Liang, and X. Jiang (2008). J. Solid State Chem. 181, 3243.
S. H. Yang, G. B. Li, S. T. Tian, F. H. Liao, and J. H. Lin (2007). Cryst. Growth. Des. 7, 1246.
C. Y. Pan, G. M. Wang, S. T. Zheng, and G. Y. Yang (2007). J. Solid State Chem. 180, 1553.
D. M. Schubert, M. Z. Visi, S. Khan, and C. B. Knobler (2008). Inorg. Chem. 47, 4740.
M. Z. Visi, C. B. Knobler, J. J. Owen, Khan Owen, M. I. Khan, and D. M. Schubert (2006). Cryst. Growth. Des. 6, 538.
W. J. Zhang and Z. H. Liu (2006). Z. Kristallogr. NCS. 221, 189.
G. Z. Liu, S. T. Zheng, and G. Y. Yang (2007). Inorg. Chem. Commun. 10, 84.
M. Li, J. Z. Chang, Z. L. Wang, and H. Z. Shi (2006). J. Solid State Chem. 179, 3265.
D. M. Schubert, M. Z. Visi, and C. B. Knobler (2008). Inorg. Chem. 47, 2017.
P. Li and Z. H. Liu (2009). Chin. J. Chem. 27, 2183.
L. Z. Wu, L. Cheng, and G. Y. Yang (2013). J. Clust. Sci.. doi:10.1007/s10876-013-0577-0.
J. Liang, Y. G. Wang, Y. X. Wang, F. H. Liao, and J. H. Lin (2013). J. Solid State Chem. 200, 99.
Y. Yang, J. B. Sun, M. Cui, R. B. Liu, Y. Wang, and C. G. Meng (2011). J. Solid State Chem. 184, 1666.
W. J. Luo, Y. G. Wang, T. Wen, H. Zhang, X. H. Lin, Y. X. Wang, F. H. Liao, and J. H. Lin (2013). J. Mater. Chem. 1048, 1.
M. S. Wang, G. C. Guo, W. T. Chen, G. Xu, W. W. Zhou, K. J. Wu, and J. S. Huang (2007). Angew. Chem. Int. Ed. 46, 3909.
G. M. Sheldrick SHELXTL 97, Program for Crystal Structure Refinements (University of Göttingen, Göttingen, 1997).
G. M. Sheldrick SHELXS97, Program for Crystal Structure Solution (University of Göttingen, Göttingen, 1997).
G. M. Sheldrick SADABS Program for Siemens Area Detector Absorption Corrections (University of Göttingen, Göttingen, 1997).
J. Li, S. P. Xia, and S. Y. Gao (1995). Spectrochim. Acta. 51A, 519.
G. Heller (1986). Top. Curr. Chem. 131, 39.
C. L. Christ and J. R. Clark (1977). Phys. Chem. Miner. 2, 59.
M. Touboul, N. Penin, and G. Nowogrocki (2003). Solid State Sci. 5, 1327.
Z. H. Liu and L. Q. Li (2006). Cryst. Growth Des. 6, 1247.
Acknowledgments
The authors are thankful for the financial supports from the China (Nos. 20871078 and 21173143), the Research Program of science and technology at Universities of Inner Mongolia Autonomous Region of China (Nos. NJZY13284 and NJZC13283), and the National Natural Science Foundation of Inner Mongolia Autonomous Region (No. 2013MS0202). The Project (No. 20130312) is supported by Inner Mengolia Science & Technology Plan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, P., Li, LQ., Huang, HS. et al. Solvothermal Syntheses and Crystal Structures of Two Novel Borates: [(CH3)3NH][B5O6(OH)4] and Na2[H2TMED][B7O9(OH)5]2 . J Clust Sci 25, 893–903 (2014). https://doi.org/10.1007/s10876-013-0663-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-013-0663-3