Abstract
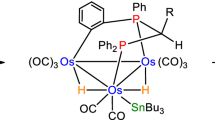
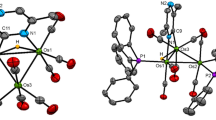
The reaction of the cluster HOs3(CO)10(μ-SC6H4Me-4) (1) with the diphosphine 4,5-bis(diphenylphosphino)-4-cyclopentadiene-1,3-dione (bpcd) has been investigated. 1 reacts with bpcd at room temperature in the presence of Me3NO to give the isomeric clusters 1,2-HOs3(CO)8(bpcd)(μ-SC6H4Me-4) (2a) and 1,1-HOs3(CO)8(bpcd)(μ-SC6H4Me-4) (2b). Clusters 2a and 2b have been isolated, and the molecular structure of each compound has been established by X-ray crystallography. The X-ray structure of 2a confirms the coordination of one of the non-hydride-bridged Os–Os vectors by the bpcd ligand, while the structure of 2b exhibits a chelating bpcd ligand that is bound to one of the osmium centers ligated by the thiolate and hydrido ligands. 2a and 2b are stable in refluxing toluene and show no evidence for bridge-to-chelate isomerization of the ancillary bpcd ligand. DFT calculations on 2a and 2b indicate that the former cluster is the thermodynamically more stable isomer. Near-UV irradiation of 2b leads to CO loss and ortho metalation of the thiolate moiety, yielding the dihydride cluster H2Os3(CO)7(bpcd)(μ,σ-SC6H3Me-4) (3). The conversion of 2b to 3 and free CO is computed to be endothermic by 14.1 kcal/mol and the reaction is driven by the entropic release of CO. The photochemically promoted ortho-metalation reaction is isomer dependent since cluster 2a is inert under identical conditions.







Similar content being viewed by others
Notes
For the activation of thiols by Os3(CO)11(MeCN) and the formation of HOs3(CO)10(μ-SR) [4].
For a report describing the equilibrium between the saturated cluster HOs3(CO)8[μ-PhP(C6H4-μ2,η1)CH2PPh2] and the kinetically active 46e cluster HOs3(CO)8[μ-PhP(C6H4-η1)CH2PPh2] see [10].
For a recent report on the isolation of HOs3(CO)8(dppm)(μ-SPh) from the reaction of HOs3(CO)8[μ-PhP(C6H4-μ2,η1)CH2PPh2] with Ph3GeSPh, see [12].
For other reactivity studies involving bpcd-substituted polynuclear clusters, see [14].
In our calculations on the osmium clusters reported here, numerous low-frequency vibrations (10–100 cm-1) were encountered in the reported stationary points. Within the harmonic oscillator approximation, these frequencies will serve as a source of error for the temperature-corrected free energy values. Accordingly, we have avoided using free energies in our discussions in the absence of corroborating experimental data [28, 29].
JIMP2, version 0.091, a free program for the visualization and manipulation of molecules [32].
These frequency assignments were also corroborated by vibrational analysis using DFT.
The observed splitting patterns for each hydride are consistent with an ABX spin system, with the hydrides assigned to the X designation within each spin system. The first-order coupling constants (2JP–H) were also successfully simulated using the program gNMR. While we cannot eliminate the possibility of spin–spin coupling between the two hydrides in cluster 3, the 2JH–H value would have to be <1 Hz.
Preliminary experiments on the carbonylation of the dihydride cluster were conducted to test the reversibility of the ortho-metalation reaction. Heating samples of cluster 3 up to 100 °C and under 34 atm CO showed no evidence of the formation of cluster 2b.
References
G. R. Crooks, B. F. G. Johnson, J. Lewis and I. G. Williams (1969). J. Chem. Soc. A 797.
R. D. Adams, J. E. Babin, and H.-S. Kim (1987). J. Am. Chem. Soc. 109, 1414.
R. D. Adams and M. P. Pompeo (1992). Organometallics 11, 2281.
K. Kiriakidou, M. R. Plutino, F. Prestopino, M. Monari, M. Johansson, L. I. Elding, E. Valls, R. Gobetto, S. Aime and E. Nordlander (1998). Chem. Commun. 2721.
E. J. Ditzel, M. P. Gómez-Sal, B. F. G. Johnson, J. Lewis and P. R. Raithby (1987). Dalton Trans. 1623.
U. Flörke, H. Egold, and M. Schraa (2000). Acta Cryst. C56, 760.
S. R. Hodge, B. F. G. Johnson, J. Lewis and P. R. Raithby (1987). Dalton Trans. 931.
S. M. T. Abedin, K. A. Azam, M. B. Hursthouse, S. E. Kabir, K. M. A. Malik, M. A. Mottalib, and E. Rosenberg (2001). J. Cluster Sci. 12, 5.
K. A. Azam, K. M. Hanif, A. C. Ghosh, S. E. Kabir, S. R. Karmakar, K. M. A. Malik, S. Parvin, and E. Rosenberg (2002). Polyhedron 21, 885.
S. -H. Huang, J. M. Keith, M. B. Hall and M. G. Richmond (2010). Organometallics 29, 4041.
K. A. Azam, S. E. Kabir, A. Miah, M. W. Day, K. I. Hardcastle, E. Rosenberg, and A. J. Deeming (1992). J. Organomet. Chem. 435, 157.
A. K. Raha, S. Ghosh, S. E. Kabir, B. K. Nicholson and D. A. Tocher (2009). J. Organomet. Chem. 694, 752.
W. H. Watson, G. Wu, and M. G. Richmond (2006). Organometallics 25, 930.
H. Shen, S. G. Bott and M. G. Richmond (1995). Organometallics 14, 4625.
S. G. Bott, H. Shen, R. A. Senter, and M. G. Richmond (2003). Organometallics 22, 1953.
S. Kandala, C. Hammons, W. H. Watson, X. Wang, and M. G. Richmond (2010). Dalton Trans. 39, 1620.
R. D. Adams and I. T. Horvath (1989). Inorg. Synth. 26, 303.
S. R. Drake and P. A. Loveday (1990). Inorg. Synth. 28, 230.
D. Fenske and H. J. Becher (1974). Chem. Ber. 107, 117.
D. F. Shriver The Manipulation of Air-Sensitive Compounds (McGraw-Hill, NY, 1969).
APEX2 Version 2.14, Bruker Advanced Analytical X-ray Systems, Inc. Copyright 2007, Madison.
G. M. Sheldrick (2008). Acta Cryst. A64, 112.
A. L. Spek (2003). J. Appl. Cryst. 36, 7.
Bruker SADABS (2007) Bruker AXS Inc., Madison.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox (2009). Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford.
A. D. Becke (1993). J. Chem. Phys. 98, 5648.
C. Lee, W. Yang, and R. G. Parr (1988). Phys. Rev. B 37, 785.
C. J. Cramer Essentials of Computational Chemistry, 2nd ed (Wiley, New York, 2004).
L. A. Watson and O. Eisenstein (2002). J. Chem. Ed. 79, 1269.
A. E. Reed, L. A. Curtiss, and F. Weinhold (1988). Chem. Rev. 88, 899.
K. B. Wiberg (1968). Tetrahedron 24, 1083.
M. B. Hall and R. F. Fenske (1972). Inorg. Chem. 11, 768.
J. Manson, C. E. Webster and M. B. Hall (2006). Texas A&M University, College Station. http://www.chem.tamu.edu/jimp2/index.html.
N. B. Colthup, L. H. Daly, and S. E. Wiberley Introduction to Infrared and Raman Spectroscopy (Academic Press, NY, 1990).
K. Yang, S. G. Bott, and M. G. Richmond (1994). Organometallics 13, 3788.
S. G. Bott, K. Yang, and M. G. Richmond (2005). J. Organomet. Chem. 690, 3067.
W. H. Watson, B. Poola, and M. G. Richmond (2006). J. Organomet. Chem. 691, 4676.
K. Biradha, V. M. Hansen, W. K. Leong, R. K. Pomeroy, and M. J. Zaworotko (2000). J. Cluster Sci. 11, 285.
P. Fompeyrine, G. Lavigne and J. -J. Bonnet (1987). Dalton Trans. 91.
X. Zhang, S. Kandala, L. Yang, W. H. Watson, X. Wang, D. A. Hrovat, W. T. Borden, and M. G. Richmond (2011). Organometallics 30, 1253.
S. W. Hunt, L. Yang, X. Wang, and M. G. Richmond (2010). J. Organomet. Chem. 696, 1432.
S. W. Hunt, V. Nesterov and M. G. Richmond (2012). J. Mol. Struct. 1010, 91.
Acknowledgments
Financial support from the Robert A. Welch Foundation (Grant B-1093-MGR) is greatly appreciated, and X. Wang acknowledges support by the U.S. Department of Energy, Office of Science, under Contract No. DE-AC05-00OR22725 managed by UT Battelle, LLC. NSF support of the NMR and computational facilities at UNT through grants CHE-0840518 and CHE-0741936 is acknowledged. MGR thanks Dr. David A. Hrovat for helpful discussions and Prof. Michael B. Hall (TAMU) for providing us a copy of his JIMP2 program, which was used to prepare the geometry-optimized structures reported here.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to our friend and colleague Professor Richard D. Adams on the occasion of his 65th birthday.
Rights and permissions
About this article
Cite this article
Yang, L., Nesterov, V.N., Wang, X. et al. CO Substitution in HOs3(CO)10(μ-SC6H4Me-4) by the Diphosphine 4,5-Bis(diphenylphosphino)-4-cyclopentadiene-1,3-dione (bpcd): Structural and DFT Evaluation of the Isomeric Clusters HOs3(CO)8(bpcd)(μ-SC6H4Me-4). J Clust Sci 23, 685–702 (2012). https://doi.org/10.1007/s10876-012-0459-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-012-0459-x