Abstract
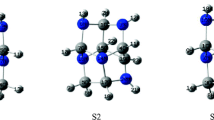
The structures, stability patterns of C26H n (n = 2) formed from the initial D 3h C26 fullerene were investigated by use of second-order-Moller–Plesset perturbation theory. The study of the stability patterns of hydrogenation reaction on C26 cage revealed that type (β) carbons were the active site and the analyses of π-orbital axis vector indicated that the reactivity of C26 was the result of the high strain and the hydrogenation reaction on C26 cage was highly exothermic. The calculated 13C NMR spectra of C26H n (n = 2) predicted that the two sp 3 hybridization carbons in C26H n (n = 2) obviously moved to high field compare with that in D 3h C26. Hence, the C26H2 should be obtained and detected experimentally. Similarly, the structures and reaction energies of C26H n (n = 4, 6, 8) were further studied at HF/6-31G*, B3LPY/6-31G* and MP2/6-31G* level. The results suggested the hydrogenation products of C26, C26H n (n = 4, 6, 8), were more stable than the C26 cage.


Similar content being viewed by others
References
R. H. Baughman, A. A. Zakhidov, and W. A. de Heer (2002). Science 297, 787.
J. J. Davis, K. S. Coleman, B. R. Azamian, C. B. Bagshaw, and M. L. H. Green (2003). Chem. Eur. J. 9, 3732.
M. Steinhart, R. B. Wehrspohn, U. Gosele, and J. H. Wendorff (2004). Angew. Chem. Int. Ed. 43, 1334.
E. Katz and I. Willner (2004). Chem-PhysChem 5, 1084.
D. M. Guldi and M. Prato (2004). Chem. Commun. 2517.
C. T. White and J. W. J. Mintmire (2005). Phys.Chem. B 109, 52.
H. W. Kroto, J. R. Heath, S. C. O’Brien, R. F. Curl, and R. E. Smalley (1985). Nature 318, 162.
W. Krätschmer, L. D. Lamb, K. Fostiropoulos, and D. R. Huffman (1990). Nature 347, 354.
H. W. Kroto (1987). Nature 329, 529.
T. G. Schmalz, W. A. Zeitz, D. J. Klein, and G. E. Hite (1988). J. Am. Chem. Soc. 110, 1113.
S. Y. Xie, F. Gao, X. Lu, R. B. Huang, C. R. Wang, X. Zhang, et al. (2004). Science 304, 699.
Y. Tan, X. Han, X. Wu, Y. Meng, F. Zhu, Z. Qian, Z. Liao, M. Chen, X. Lu, S. Xie, R. Huang, and L. Zheng (2008). J. Am. Chem. Soc. 130, 15240.
R. E. Haufler, J. Conceicao, L. P. F. Chibante, Y. Chai, N. E. Byrne, S. Flanagan, et al. (1990). J. Phys. Chem. 94, 8634.
L. Becker, T. P. Evans, and J. L. Bada (1993). J. Org. Chem. 58, 7630.
M. I. Attalla, A. M. Vassallo, B. N. Tattam, and J. V. Hanna (1993). J. Phys. Chem. 97, 6329.
C. Rüchardt, M. Gerst, J. Ebenhoch, H.-D. Beckhaus, E. E. B. Campbell, R. Tellgmann, et al. (1993). Angew. Chem. Int. Ed. Engl. 32, 584.
C. C. Henderson and P. A. Cahill (1993). Science 259, 1885.
C. Jin, R. Hettich, R. Compton, D. Joyce, J. Blencoe, and T. Burch (1994). J. Phys. Chem. 98, 4215.
D. Mandrus, M. Kele, R. L. Hettich, G. Guiochon, B. C. Sales, and L. A. A. Boatner (1997). J. Phys. Chem. B 101, 123.
N. Matsuzawa, D. A. Dixon, and T. Fukunaga (1992). J. Phys. Chem. 96, 7594.
C. R. Wang, Z. Q. Shi, L. J. Wan, X. Lu, L. Dunsch, C. Y. Shu, et al. (2006). J. Am. Chem. Soc. 128, 6605.
A. Koshio, M. Inakuma, T. Sugai, and H. Shinohara (2000). J. Am. Chem. Soc. 122, 398.
H. Prinzbach (1993). Angew. Chem. Int. Ed. Engl. 32, 1722.
H. Prinzbach and K. Weber (1994). Angew. Chem. Int. Ed. Engl. 33, 2239.
P. R. C. Kent, M. D. Towler, R. J. Needs, and G. Rajagopal (2000). Phys. Rev. B 62, 15394.
C. Moller and M. S. Plesset (1934). Phys. Rev. 46, 618.
R. C. Haddon (1993). Science 261, 1545.
R. C. Haddon (2001). J. Phys. Chem. A 105, 4164.
A. D. Becke (1993). J. Chem. Phys. 98, 5648.
C. Lee, W. Yang, and R. G. Parr (1988). Phys. Rev. B 37, 785.
T. E. Gunda MOL2MOL, version 5.3 (University of Debrecen, Debrecen, Hungary, 2004).
M. J. Frisch, G. W. Trucks, G. W. Trucks, H. B. Schlegel, et al. GAUSSIAN03 (Gaussian, Inc., Pittsburgh PA, 2003).
B. Paulus (2003). Phys. Chem. Chem. Phys. 5, 3364.
Acknowledgments
The authors thank National Natural Science Foundation of China (NSFC) (Grant No. 40871107), Eleventh Five-year Plan Research Project of Jilin Province Education Department (Grants 2008305) and Doctor Research Starting Fund of Jilin Agriculture University (Grants 2007005) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hong, B., Chang, YF., Sun, LL. et al. Theoretical Study of the Structures, Properties and Spectroscopies on Fullerene Hydrides C26H n (n = 2, 4, 6, 8). J Clust Sci 22, 1–10 (2011). https://doi.org/10.1007/s10876-011-0361-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-011-0361-y