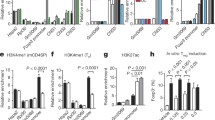
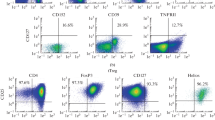
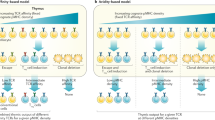
Abstract
Foxp3-expressing regulatory T cells (Treg) have an essential function of preventing autoimmune disease in man and mouse. Foxp3 binds to forkhead motifs of about 1,100 genes and the strength of binding increases upon phorbol 12-myristate 13-acetate/ionomycin stimulation. In Foxp3-expressing T cell hybridomas, Foxp3 promoter binding does not lead to activation or suppression of genes which becomes only visible after T cell activation. These findings are in line with observations by others that Foxp3 exerts important functions in collaboration with T cell receptor (TCR)-dependent transcription factors in a DNA-binding complex. Tregs can be generated when developing T cells encounter TCR agonist ligands in the thymus. This process apparently depends on costimulatory signals. In contrast, extrathymic conversion of naïve T cells into Tregs appears to depend on transforming growth factor (TGF)-β and is inhibited by costimulation. In fact, dendritic cell-derived retinoic acid helps the conversion process by counteracting the negative impact of costimulation. Tregs induced by subimmunogenic antigen delivery in vivo are much more stable than Tregs induced by antigenic stimulation in the presence of TGF-β in vitro which correlates with the extent of demethylation of the Foxp3 locus. Tregs can be induced by conversion of antigen-specific T cells that occur with a very low frequency in wt mice. Conversion of naïve cluster of differentiation (CD)4 T cells into Tregs by a single peptide of HY antigens results in complete antigen-specific tolerance to an entire set of HY epitopes recognized by CD4 as well as CD8 T cells when presented with male skin or hemopoietic grafts.
Similar content being viewed by others
References
Itoh M, Takahashi T, Sakaguchi N, et al. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol 1999;162:5317–26.
Bruder D, Probst-Kepper M, Westendorf AM, et al. Neuropilin-1: a surface marker of regulatory T cells. Eur J Immunol 2004;34:623–30.
Huehn J, Siegmund K, Lehmann JC, et al. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med 2004;199:303–13.
Hansen W, Loser K, Westendorf AM, et al. G protein-coupled receptor 83 overexpression in naive CD4+CD25- T cells leads to the induction of Foxp3+ regulatory T cells in vivo. J Immunol 2006;177:209–15.
Shimizu J, Yamazaki S, Takahashi T, et al. Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol 2002;3:135–4.
Bachmann MF, Kohler G, Ecabert B, et al. Cutting edge: lymphoproliferative disease in the absence of CTLA-4 is not T cell autonomous. J Immunol 1999;163:1128–31.
Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with Foxp3 and suppressive function of human CD4+ Treg cells. J Exp Med 2006;203:1701–11.
Kretschmer K, Apostolou I, Hawiger D, et al. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol 2005;6:1219–27.
Fontenot JD, Rasmussen JP, Williams LM, et al. Regulatory T cell lineage specification by the forkhead transcription factor Foxp3. Immunity 2005;22:329–41.
Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci USA 2005;102:5126–31.
Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003;4:330–6.
Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003;299:1057–61.
Jaeckel E, von Boehmer H, Manns MP. Antigen-specific FoxP3-transduced T-cells can control established type 1 diabetes. Diabetes 2005;54:306–10.
Tang Q, Henriksen KJ, Bi M, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med 2004;199:1455–65.
Tarbell KV, Yamazaki S, Olson K, et al. CD25+CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med 2004;199:1467–77.
Wu Y, Borde M, Heissmeyer V, et al. Foxp3 controls regulatory T cell function through cooperation with NFAT. Cell 2006;126:375–87.
Gavin MA, Rasmussen JP, Fontenot JD, et al. Foxp3-dependent programme of regulatory T-cell differentiation. Nature 2007;445:771–5.
Marson A, Kretschmer K, Frampton GM, et al. Foxp3 occupancy and regulation of key target genes during T-cell stimulation. Nature 2007;445:931–5.
Klein L, Khazaie K, von Boehmer H. In vivo dynamics of antigen-specific regulatory T cells not predicted from behavior in vitro. Proc Natl Acad Sci USA 2003;100:8886–91.
von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol 2005;6:338–44.
von Boehmer H. Selection of the T-cell repertoire: receptor-controlled checkpoints in T-cell development. Adv Immunol 2004;84:201–38.
Jordan MS, Boesteanu A, Reed AJ, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol 2001;2:301–6.
Apostolou I, Sarukhan A, Klein L, et al. Origin of regulatory T cells with known specificity for antigen. Nat Immunol 2002;3:756–63.
Tai X, Cowan M, Feigenbaum L, et al. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat Immunol 2002;6:152–62.
Vafiadis P, Bennett ST, Todd JA, et al. Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nat Genet 1997;15:289–9.
Derbinski J, Schulte A, Kyewski B, et al. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol 2001;2:1032–9.
Aschenbrenner K, D’Cruz LM, Vollmann EH, et al. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol 2007;8:351–8.
Liston A, Gray DH, Lesage S, et al. Gene dosage—limiting role of Aire in thymic expression, clonal deletion, and organ-specific autoimmunity. J Exp Med 2004;200:1015–26.
Anderson MS, Venanzi ES, Chen Z, et al. The cellular mechanism of Aire control of T cell tolerance. Immunity 2005;23:227–39.
Hao Y, Legrand N, Freitas AA. The clone size of peripheral CD8 T cells is regulated by TCR promiscuity. J Exp Med 2006;203:1643–9.
Kretschmer K, Apostolou I, Jaeckel E, et al. Making regulatory T cells with defined antigen specificity: role in autoimmunity and cancer. Immunol Rev 2006;212:163–9.
Apostolou I, Von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J Exp Med 2004;199:1401–8.
Polansky JK, Kretschmer K, Freyer J, Floess S, Garbe A, Baron U, Olek S, Hamann A, von Boehmer H, Huehn J. DNA methylation controls Foxp3 gene expression. Eur J Immunol 2008;38:1654–6.
Verginis P, McLaughlin KA, Wucherpfennig KW. Induction of antigen-specific regulatory T cells in wild-type mice: visualization and mode of suppression. Proc Natl Acad Sci USA 2008;105:3479–84.
Benson MJ, Pino-Lagos K, Rosemblatt M, et al. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med 2007;204:1765–74.
Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med 2007;204:1757–64.
Sun CM, Hall JA, Blank RB, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 Treg cells via retinoic acid. J Exp Med 2007;204:1775–85.
von Boehmer H. Oral tolerance: is it all retinoic acid? J Exp Med 2007;204:1737–9.
Mempel TR, Pittet MJ, Khazaie K, et al. Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation. Immunity 2006;25:129–41.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Apostolou, I., Verginis, P., Kretschmer, K. et al. Peripherally Induced Treg: Mode, Stability, and Role in Specific Tolerance. J Clin Immunol 28, 619–624 (2008). https://doi.org/10.1007/s10875-008-9254-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-008-9254-8