Abstract
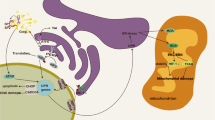
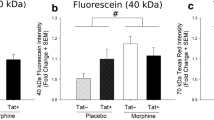
Human immunodeficiency virus (HIV)-1 patients who abuse opiates are at a greater risk of developing neurological complications of AIDS. Alterations in blood–brain barrier (BBB) integrity are associated with cytoskeletal disorganization and disruption of tight junction (TJ) integrity. We hypothesize that opiates in combination with HIV-1 viral proteins can modulate TJ expression in primary brain microvascular endothelial cells (BMVEC), thereby compromising BBB integrity and exacerbating HIV-1 neuropathogenesis. We investigated the effect of morphine and/or tat on the expression of TJ proteins ZO-1, JAM-2, Occludin and P-glycoprotein and the functional effects of TJ modulation in BMVEC. Morphine and/or tat, via the activation of pro-inflammatory cytokines, intracellular Ca2+ release, and activation of myosin light chain kinase, modulated TJ expression resulting in decreased transendothelial electric resistance and enhanced transendothelial migration across the BBB. These studies may lead to the development of novel anti-HIV-1 therapeutics that target specific TJ proteins, thus preventing TJ disruption in opiate using HIV-1 patients.





Similar content being viewed by others
References
Ozdener H. Molecular mechanisms of HIV-1 associated neurodegeneration. J Biosci 2005;30(3):391–405.
Trujillo JR, Jaramillo-Rangel G, Ortega-Martinez M, Penalva de Oliveira AC, Vidal JE, Bryant J, et al. International neuroAIDS: prospects of HIV-1 associated neurological complications. Cell Res 2005;15(11–12):962–9.
Lipton SA. HIV-related neurotoxicity. Brain Pathol 1991;1:193.
Nottet HS, Persidsky Y, Sasseville VG, Nukuna AN, Bock P, Zhai QH, et al. Mechanisms for the transendothelial migration of HIV-1-infected monocytes into brain. J Immunol 1996;156:1284.
Nottet HS. Interactions between macrophages and brain microvascular endothelial cells: role in pathogenesis of HIV-1 infection and blood–brain barrier function. J Neurovirol 1999;5:659.
Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis 2002;186(Suppl 2):S193.
Gendelman HE, Persidsky Y, Ghorpade A, Limoges J, Stins M, Fiala M, et al. The neuropathogenesis of the AIDS dementia complex. AIDS 1997;11(Suppl A):S35.
Persidsky Y, Ghorpade A, Rasmussen J, Limoges J, Liu XJ, Stins M, et al. Microglial and astrocyte chemokines regulate monocyte migration through the blood–brain barrier in human immunodeficiency virus-1 encephalitis. Am J Pathol 1999;155:1599.
Boven LA, Middel J, Breij EC, Schotte D, Verhoef J, Soderland C, et al. Interactions between HIV-infected monocyte-derived macrophages and human brain microvascular endothelial cells result in increased expression of CC chemokines. J Neurovirol 2000;6:382.
Krebs FC, Ross H, McAllister J, Wigdahl B. HIV-1-associated central nervous system dysfunction. Adv Pharmacol 2000;49:315.
Wu DT, Woodman SE, Weiss JM, McManu CM, D’Aversa TG, Hesselgesser J, et al. Mechanisms of leukocyte trafficking into the CNS. J Neurovirol 2000;6(Suppl 1):S82.
Worthylake RA, Burridge K. Leukocyte transendothelial migration: orchestrating the underlying molecular machinery. Curr Opin Cell Biol 2001;13:569.
Kolson DL. Neuropathogenesis of central nervous system HIV-1 infection. Clin Lab Med 2002;22:703.
Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood–brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci 2006;26(4):1098–106.
Chang SL, Felix B, Jiang Y, Fiala M. Actions of endotoxin and morphine. Adv Exp Med Biol 2001;493:187–96.
Mahajan SD, Schwartz SA, Shanahan TC, Chawda RP, Nair MP. Morphine regulates gene expression of alpha- and beta-chemokines and their receptors on astroglial cells via the opioid mu receptor. J Immunol 2002;169(7):3589–99.
Mahajan SD, Aalinkeel R, Reynolds JL, Nair BB, Fernandez SF, Schwartz SA, et al. Morphine exacerbates HIV-1 viral protein gp120 induced modulation of chemokine gene expression in U373 astrocytoma cells. Curr HIV Res 2005;3(3):277–88.
Nath A, Anderson C, Jones M, Maragos W, Booze R, Mactutus C, et al. Neurotoxicity and dysfunction of dopaminergic systems associated with AIDS dementia. J Psychopharmacol 2000;14(3):222–7.
Li W, Galey D, Mattson MP, Nath A. Molecular and cellular mechanisms of neuronal cell death in HIV dementia. Neurotoxicol Res 2005;8(1–2):119–34.
Donahoe RM, Vlahov D. Opiates as potential cofactors in progression of HIV-1 infections to AIDS. J Neuroimmunol 1998;83(1–2):77–87.
Hauser KF, El-Hage N, Buch S, Berger JR, Tyor WR, Nath A, et al. Molecular targets of opiate drug abuse in neuroAIDS. Neurotox Res 2005;8(1–2):63–80.
Hauser KF, El-Hage N, Buch S, Nath A, Tyor WR, Bruce-Keller AJ, et al. Impact of opiate-HIV-1 interactions on neurotoxic signaling. J Neuroimmune Pharmacol 2006;1(1):98–105.
Balda MS, Whitney JA, Flores C, Gonzalez S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol 1996;134(4):1031–49.
Balda M, Matter K. Tight junctions. J Cell Sci 1998;111(5):541–7.
Ma TY, Tran D, Hoa N, Nguyen D, Merryfield M, Tarnawski A. Mechanism of extracellular calcium regulation of intestinal epithelial tight junction permeability: role of cytoskeletal involvement. Microsc Res Tech 2000;51:156.
Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood–brain barrier. Trends Neurosci 2001;24:719.
Aurrand-Lions M, Duncan L, Ballestrem C, Imhof BA. JAM-2, a novel immunoglobulin superfamily molecule, expressed by endothelial and lymphatic cell. J Biol Chem 2001;276(4):2733–41.
Aurrand-Lions M, Johnson-Leger C, Lamagna C, Ozaki H, Kita T, Imhof BA. Junctional adhesion molecules and interendothelial junctions. Cells Tissues Organs 2002;172(3):152–60.
Luscinskas FW, Ma S, Nusrat A, Parkos CA, Shaw SK. The role of endothelial cell lateral junctions during leukocyte trafficking. Immunol Rev 2002;186:57.
Andras IE, Pu H, Deli MA, Nath A, Hennig B, Toborek M. HIV-1 Tat protein alters tight junction protein expression and distribution in cultured brain endothelial cells. J Neurosci Res 2003;74:255–65.
Andras IE, Pu H, Tian J, Deli MA, Nath A, Hennig B, et al. Signaling mechanisms of HIV-1 Tat-induced alterations of claudin-5 expression in brain endothelial cells. J Cereb Blood Flow Metab 2005;25(9):1159–70.
Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol 2004;286(6):C1213–28.
Hawkins BT, Davis TP. The blood–brain barrier/neurovascular unit in health and disease. Pharmacol Rev 2005;57(2):173–85.
Denker BM, Nigam SK. Molecular structure and assembly of the tight junction. Am J Physiol Renal Physiol 1998;274:F1–F9.
Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol 1998;141(7):1539–50.
Furuse M, Sasaki H, Tsukita S. Manner of interaction of heterogeneous claudin species within and between tight junction strands. J Cell Biol 1999;147:891.
Bazzoni G, Martinez-Estrada OM, Orsenigo F, Cordenonsi M, Citi S, Dejana E. Interaction of junctional adhesion molecule with the tight junction components ZO-1, cingulin, and occludin. J Biol Chem 2000;275(27):20520–6.
Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev 2006;86(1):279–367.
Tsukita S, Furuse M, Itoh M. Molecular architecture of tight junctions: occludin and ZO-1. Soc Gen Physiol Ser 1997;52:69–76.
Pu H, Tian J, Flora G, Lee YW, Nath A, Hennig B, Toborek M. HIV-1 Tat protein upregulates inflammatory mediators and induces monocyte invasion into the brain. Mol Cell Neurosci 2003;24(1):224–37.
Cabral GA. Drugs of abuse, immune modulation, and AIDS. J Neuroimmune Pharmacol 2006;1(3):280–95.
Toborek M, Lee YW, Flora G, Pu H, Andras IE, Wylegala E, et al. Mechanisms of the blood–brain barrier disruption in HIV-1 infection. Cell Mol Neurobiol 2005;25(1):181–99.
Persidsky Y, Gendelman HE. Development of laboratory and animal model systems for HIV-1 encephalitis and its associated dementia. J Leukoc Biol 1997;62(1):100–6.
Persidsky Y, Stins M, Way D, Witte MH, Weinand M, Kim KS, et al. A model for monocyte migration through the blood–brain barrier during HIV-1 encephalitis. J Immunol 1997;158(7):3499–510.
Mahajan S, Schwartz SA, Sykes D, Chawda R, Aalinkeel R, Nair MPN. Effect of HIV peptides on trans-endothelial migration of dendritic cells across the blood brain barrier. 12th International Congress of Immunology and 4th Annual Conference of FOCIS, Montreal, Canada, July 2004, Medimond Press, p. 153–159, 2004.
Meyer TP, Zehnter I, Hofmann B, Zaisserer J, Burkhart J, Rapp S, et al. Filter buffy coats (FBC): a source of peripheral blood leukocytes recovered from leukocyte depletion filters. J Immunol Methods 2005;307(1–2):150–66.
Banks WA, Kastin AJ, Akerstrom V. HIV-1 protein gp120 crosses the blood–brain barrier: role of adsorptive endocytosis. Life Sci 1997;61:PL119–25.
Ricardo-Dukelow M, Kadiu I, Rozek W, Schlautman J, Persidsky Y, Ciborowski P, et al. HIV-1 infected monocyte-derived macrophages affect the human brain microvascular endothelial cell proteome: new insights into blood–brain barrier dysfunction for HIV-1-associated dementia. J Neuroimmunol 2007;185(1–2):37–46.
Chomczynski P, Saachi N. Single step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987;162:156–9.
Bustin SA. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J Mol Endocrinol 2002;29(1):23–39.
Radonić A, Thulke S, Mackay IM, Landt O, Siegert W, Nitsche A. Guideline to reference gene selection for quantitative real-time PCR. Biochem Biophys Res Commun 2004;313(4):856–62.
Current Protocols in Immunology, CopyrightÓ 2007 by Wiley, Hoboken, NJ. 2007.
Fernandez SF, Huang MH, Davidson BA, Knight PR 3rd, Izzo JL Jr. Mechanisms of angiotensin II-mediated decreases in intraneuronal Ca2+ in calcium-loaded stellate ganglion neurons. Hypertension 2005;45(2):276–82.
Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD. Blood–brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol 2006;1(3):223–36.
Marie-Claire C, Courtin C, Roques BP, Noble F. Cytoskeletal genes regulation by chronic morphine treatment in rat striatum. Neuropsychopharmacology 2004;29(12):2208–15.
Khurdayan VK, Buch S, El-Hage N, Lutz SE, Goebel SM, Singh IN, et al. Preferential vulnerability of astroglia and glial precursors to combined opioid and HIV-1 tat exposure in vitro. Eur J Neurosci 2004;19(12):3171–82.
Collins NT, Cummins PM, Colgan OC, Ferguson G, Birney YA, Murphy RP, et al. Cyclic strain-mediated regulation of vascular endothelial occludin and ZO-1: influence on intercellular tight junction assembly and function. Arterioscler Thromb Vasc Biol 2006;26(1):62–8.
Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol 1998;142:117.
Ryan LA, Brester M, Bohac D, Morgello S, Zheng J. Up-regulation of soluble tumor necrosis factor receptor two in plasma of HIV-seropositive individuals who use opiates. AIDS Res Hum Retroviruses 2004;20(1):41–5.
Xie R, et al. The role of P-glycoprotein in blood–brain barrier transport of morphine: transcortical microdialysis studies in mdr1a (−/−) and mdr1a (+/+) mice. Br J Pharmacol 1999;128(3):563–8.
Aquilante CL, Letrent SP, Pollack GM, Brouwer KL. Increased brain P-glycoprotein in morphine tolerant rats. Life Sci 2000;66(4):PL47–51.
Hayashi K, Pu H, Tian J, Andras IE, Lee YW, Hennig B, et al. HIV-tat protein induces P-glycoprotein expression in brain microvascular endothelial cells. J Neurochem 2005;93(5):1231–41.
Hayashi K, Pu H, Andras IE, Eum SY, Yamauchi A, Hennig B, et al. HIV-TAT protein upregulates expression of multidrug resistance protein 1 in the blood–brain barrier. J Cereb Blood Flow Metab 2006;26(8):1052–65.
Yu C, Kastin AJ, Tu H, Waters S, Pan W. TNF activates P-glycoprotein in cerebral microvascular endothelial cells. Cell Physiol Biochem 2007;20(6):853–8.
Wong D, Dorovini-Zis K, Vincent SR. Cytokines, nitric oxide, and cGMP modulate the permeability of an in vitro model of the human blood–brain barrier. Exp Neurol 2004;190 (2):446–55.
El-Hage N, Gurwell JA, Singh IN, Knapp PE, Nath A, Hauser KF. Synergistic increases in intracellular Ca2+, and the release of MCP-1, RANTES, and IL-6 by astrocytes treated with opiates and HIV-1 tat. Glia 2005;50(2):91–106.
Nath A, Psooy K, Martin C, Knudsen B, Magnuson DS, Haughey N, et al. Identification of a human immunodeficiency virus type 1 tat epitope that is neuroexcitatory and neurotoxic. J Virol 1996;70(3):1475–80.
Haorah J, Heilman D, Knipe B, Chrastil J, Leibhart J, Ghorpade A, et al. Ethanol-induced activation of myosin light chain kinase leads to dysfunction of tight junctions and blood–brain barrier compromise. Alcohol Clin Exp Res 2005;29(6):999–1009.
Ishmael JE, Löhr CV, Fischer K, Kioussi C. Localization of myosin II regulatory light chain in the cerebral vasculature. Acta Histochem 2008;110:172–7.
Acknowledgments
This work was supported in part by grants from the Margaret Duffy and Robert Cameron Troup Memorial Fund of Kaleida Health and the Kaleida Health Foundation. Authors would like to thank Elizabeth Hemedinger, Manager of Laboratory Services at Upstate New York Transplant Services, 110 Broadway, Buffalo, NY 14203 for her assistance in providing leukocyte depletion filters as source of peripheral leukocyte populations for these studies.
Author information
Authors and Affiliations
Corresponding author
Appendix: Abbreviations
Appendix: Abbreviations
- HIV-1:
-
human immunodeficiency virus-1
- ADC:
-
AIDS dementia complex
- BBB:
-
blood–brain barrier
- BMVEC:
-
brain microvascular endothelial cells
- CNS:
-
central nervous system
- DEPC:
-
diethyl pyrocarbonate
- HAD:
-
HIV-1-associated dementia
- HIVE:
-
human immunodeficiency virus associated encephalopathy
- IL-8:
-
Interleukin-8
- JAM-2:
-
junctional adhesion molecule-2
- MLCK:
-
myosin light chain kinase
- NHA:
-
normal human astrocytes
- PBS:
-
phosphate buffer saline
- TAI:
-
transcript accumulation index
- TEER:
-
transendothelial electrical resistance
- TJ:
-
tight junctions
- TNF-α:
-
tumor necrosis factor-alpha
- ZO-1:
-
zona occludins
Rights and permissions
About this article
Cite this article
Mahajan, S.D., Aalinkeel, R., Sykes, D.E. et al. Tight Junction Regulation by Morphine and HIV-1 Tat Modulates Blood–Brain Barrier Permeability. J Clin Immunol 28, 528–541 (2008). https://doi.org/10.1007/s10875-008-9208-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-008-9208-1