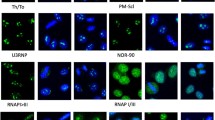
The most important mitotic apparatus (MA) antigens are centrosome (CE), nuclear mitotic apparatus (NuMA-1, NuMA-2), midbody, and centromere F (CENP-F). We studied associations of anti-MA antibodies with other autoantibodies and their clinical significance. A total of 6270 patients were studied for the presence of anti-MA antibodies on HEp-2 cells. Sera positive for anti-MA were tested for anti-extractable nuclear antigens (ENA) antibodies. Anti-MA antibodies were detected in 56 (45 females and 11 males) of 6270 sera (0.9%). Of these 56, NuMA-1 was found in 23, NuMA-2 in 7, CE in 20, CENP-F in 5, and CENP-F/centrosome in 1 case. Anti-NuMA-1 were associated with anti-ENA antibodies (p < 0.001). Diagnoses were established in 43/56 patients: 22 connective tissue diseases, 7 infections, 6 autoimmune hepatitis, 3 vasculitis, 3 primary antiphospholipid syndrome, 1 malignancy, and 1 fever of unknown origin. The differential diagnosis of anti-NuMA-1-positive patients must include Sjögren’s syndrome, while patients with anti-CE antibodies must be observed for HCV infection.




Similar content being viewed by others
REFERENCES
Fritzler MJ, Rattner JB: Autoantibodies to the mitotic apparatus: Biological breakthroughs, clinical application, etiological complexity. In Autoantigens and Autoantibodies: Diagnostic Tools and Clues to Understanding Autoimmunity, K Conrad, RL Humbel, et al. (eds). Berlin, Pabst Science, 2000, pp 58–86
Ou Y, Rattner JB: A subset of centrosomal proteins are arranged in a tubular conformation that is reproduced during centrosome duplication. Cell Motil Cytoskeleton 47:13–24, 2000
Kisurina-Evgenieva O, Mack G, Du Q, Macara I, Khodjakov A, Compton DA: Multiple mechanisms regulate NuMA dynamics at spindle poles. J Cell Sci 117:6391–6400, 2004
Bradwell AR, Hughes RG, Harden EL: Atlas of HE-p 2 Patterns. Birmingham, Drapkins, 2003
Casiano CA, Martin SJ, Green DR, Tan EM: Selective cleavage of nuclear autoantigens during CD95 (Fas/APO-1)-mediated T cell apoptosis. J Exp Med 184:765–770, 1996
Ramirez-Sandoval R, Sanchez-Rodriguez SH, Herrera-van Oostdam D, Avalos-Diaz E, Herrera-Esparza R: Antinuclear antibodies recognize cellular autoantigens driven by apoptosis. Joint Bone Spine 70:187–194, 2003
Casciola-Rosen LA, Anhalt G, Rosen A: Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface blebs on apoptotic keratinocytes. J Exp Med 179:1317–1330, 1994
Andrade LE, Chan EK, Peebles CL, Tan EM: Two major autoantigen–antibody systems of the mitotic spindle apparatus. Arthritis Rheum 39:1643–1653, 1996
Huidbuchel E, Blaschek M, Seigneurin JM, Lamour A, Berthelot JM, Youinou P: Anti-organelle and anti-cytoskeletal autoantibodies in the serum of Epstein-Barr virus-infected patients. Ann Med Intern (Paris) 142:343–346, 1991
Abu-Shakra M, Buskila D, Ehrenfeld M, Conrad K, Shoenfeld Y: Cancer and autoimmunity: autoimmune and rheumatic features in patients with malignancies. Ann Rheum Dis 60:433–441, 2001
Hochberg MC: Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40:1725, 1997
Sharp GC: Diagnostic criteria for classification of MCTD. In Mixed Connective Tissue Diseases and Antinuclear Antibodies, R Kasukawa, GC Sharp (eds). Amsterdam, Elsevier, 1987, pp 23–32
Anonymous: Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association diagnostic and therapeutic criteria committee. Arthritis Rheum 23:581–590, 1980
Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, Daniels TE, Fox PC, Fox RI, Kassan SS, Pillemer SR, Talal N, Weisman MH: European Study Group on Classification Criteria for Sjogren’s Syndrome. Classification criteria for Sjogren’s syndrome: A revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 61:554–558, 2002
Bohan A, Peter JB: Polymyositis and dermatomyositis. N Engl J Med 292:344–347, 1975
Mosca M, Neri R, Stringini F, Carmignani A, Totti D, Tavoni A, Bombardieri S: Pregnancy outcome in patients with undifferentiated connective tissue disease: A preliminary study on 25 pregnancies. Lupus 11:304–307, 2002
Wilson WA, Gharavi AE, Koike T, Lockshin MD, Branch DW, Piette JC, Brey R, Derksen R, Harris EN, Hughes GR, Triplett DA, Khamashta MA: International consensus statement on preliminary classification for definite antiphospholipid syndrome. Arthritis Rheum 42:1309–1311, 1999
Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, et al.: International autoimmune hepatitis group report: Review of criteria for diagnosis of autoimmune hepatitis. J Hepatol 31:929–938, 1999
Jennette JC, Falk RJ: Small-vessels vasculitis. N Engl J Med 20:1512–1523, 1997
Radice A, Vecchi M, Bianchi MB, Sinico RA: Contribution of immunoflorescence to the identification and characterization of anti-neutrophil cytoplasmic autoantibodies. The role of different fixatives. Clin Exp Rheum 18:707–1712, 2000
Auer-Grumbach P, Achleitner B: Epidemiology and clinical associations of NuMA (Nuclear Mitotic Apparatus Protein) autoantibodies. J Rheumatol 21:1779–1781, 1994
Fritzler MJ, Pauls JD, Kinsella TD, Bowen TJ: Antinuclear, anticytoplasmic and anti Sjogren syndrome antigen A (SS-A/Ro) antibodies in female blood donors. Clin Immunol Immunopathol 36:120–128, 1985
Taimen P, Viljamaa M, Kallajoki M: Preferential expression of NuMA in the nuclei of proliferating cells. Exp Cell Res 256:140–149, 2000
Kammerer S, Roth RB, Hoyal CR, Reneland R, Marnellos G, Kiechle M, et al.: Association of the NuMA region on chromosome 11q13 with breast cancer susceptibility. Proc Natl Acad Sci USA 102:2004–2009, 2005
Yang CH, Lambie EJ, Snyder M: NuMA: An unusually long coiled-coil related protein in the mammalian nucleus. J Cell Biol 116:1303–1317, 1992
Whitehead CM, Winkfein RJ, Fritzler MJ, Rattner JB: The spindle kinesin-like protein HsEg5 is an autoantigen in systemic lupus erythematosus. Arthritis Rheum 39:1635–1642, 1996
Rattner JB, Martin L, Waisman DM, Johnstone SA, Fritzler MJ: Autoantibodies to the centrosome (centrosome) react with determinants present in the glycolytic enzyme enolase. J Immunol 146:2341–2344, 1991
Liao H, Winkfein RJ, Mack G, Rattner JB, Yen TJ: CENP-F is a protein of the nuclear matrix that assembles onto kinetochores at late G2 and is rapidly degraded after mitosis. J Cell Biol 130:507–518, 1995
Casiano CA, Landberg G, Ochs RL, Tan EM: Autoantibodies to a novel cell cycle-regulated protein that accumulates in the nuclear matrix during S phase and is localized in the kinetochores and spindle midzone during mitosis. J Cell Sci 106:1045–1056, 1993
Hansen BU, Eriksson S, Lindgren S: High prevalence of autoimmune liver disease in patients with multiple nuclear dot, anti-centromere, and mitotic spindle antibodies. Scand J Gastroenterol 26:707–713, 1991
Grypiotis P, Ruffatti A, Tonello M, Winzler C, Radu C, Zampieri S, et al.: Clinical significance of fluoroscopic patterns specific for the mitotic spindle in patients with rheumatic diseases. Reumatismo 54:232–237, 2002
McCarty GA, Valencia DW, Fritzler MJ: Antibody to the mitotic spindle apparatus: immunologic characteristics and cytologic studies. J Rheumatol 11:213–218, 1984
Hassfeld W, Chan EK, Mathison DA, Portman D, Dreyfuss G, Steiner G, Tan EM: Molecular definition of heterogeneous nuclear ribonucleoprotein R (hnRNP R) using autoimmune antibody: Immunological relationship with hnRNP P. Nucleic Acids Res 26:439–445, 1998
Zeng C, He D, Berget SM, Brinkley BR: Nuclear-mitotic apparatus protein: A structural protein interface between the mucleoskeleton and RNA splicing. Proc Natl Acad Sci USA 91:1505–1509, 1994
Hsu HL, Yeh NH: Dynamic changes of NuMA during the cell cycle and possible appearance of a truncated form of NuMA during apoptosis. J Cell Sci 109:277–288, 1996
Price CM, McCarty GA, Pettijohn DE: NuMA protein is a human autoantigen. Arthritis Rheum 27:774–779, 1984
Sato S, Fujimoto M, Ihn H, Takehara K: Antibodies to centromere and centrosome in scleroderma spectrum disorders. Dermatology 89:23–26, 1994
Fritzler MJ, Manns MP: Anti-mitochondrial antibodies. Clin Appl Immunol Rev 3:87–113, 2002
Ramos-Casals M, Jara LJ, Medina F, Rosas J, Calvo-Alen, Mana J, et al.: Systemic autoimmune diseases co-existing with chronic hepatitis C virus infection (the Hispamec Registry): Patterns of clinical and immunological expression in 180 cases. J Intern Med 57:549–557, 2005
Gentric A, Blaschek M, Julien C, Jouquan J, Pennec Y, Berthelot JM, et al.: Nonorgan-specific autoantibodies in individuals infected with type 1 human immunodeficiency virus. Clin Immunol Immunopathol 59:487–494, 1991
Bencimon C, Salles G, Moreira A, Guyomard S, Coiffier B, Bienvenu J, Fabien N: Prevalence of anticentromere F protein autoantibodies in 347 patients with non-Hodgkin’s lymphoma. Ann NY Acad Sci 1050:319–326, 2005
Zhang JY, Zhu W, Imai H, Kiyosawa K, Chan EK, Tan EM: De-novo humoral immune responses to cancer-associated autoantigens during transition from chronic liver disease to hepatocellular carcinoma. Clin Exp Immunol 125:3–9, 2001
Rattner JB, Rees J, Whitehead CM, Casiano CA, Tan EM, Humbel RL, et al.: High frequency of neoplasia in patients with autoantibodies to centromere protein CENP-F. Clin Invest Med 20:308–319, 1997
ACKNOWLEDGMENTS
This study was supported in part by Ministry of Science of the Republic of Serbia, Grant No. 145032 Dj.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
BONACI-NIKOLIC, B., ANDREJEVIC, S., BUKILICA, M. et al. Autoantibodies to Mitotic Apparatus: Association with Other Autoantibodies and Their Clinical Significance. J Clin Immunol 26, 438–446 (2006). https://doi.org/10.1007/s10875-006-9038-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-006-9038-y