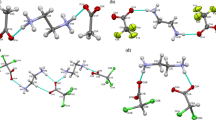
Abstract
Lansoprazole sulphide (2-[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl]methylthio-1H-benzimidazole) hydrate crystallizes in the triclinic space group P-1 with two molecules in the asymmetric part of the unit cell. The molecules are almost identical, the normal probability plots show that the differences between them are of statistical nature. The crystal structure is determined mainly by the O–H···N and N–H···O hydrogen bonds; and both symmetry independent molecules create the hydrogen-bonded structures on their own. The common motif is the C 22 (6) chain of molecules along x (A) or y (B), but the interactions between the chains are different: chains of molecules A are joined by O–H···N(pyridine) hydrogen bonds while those of molecules B– by relatively strong O–H···S hydrogen bonds. Additionally, in both cases there are also C–H···S, C–H···π and π–π interactions between the neighbouring molecules. The different intermolecular interactions might be connected with the observed disorder of water molecules in the B-chains. At room temperature the s.o.f.’s of two alternative positions refined at 0.760(17) and 0.240(17). The less-occupied water molecule does not take part in the O–H···S hydrogen bonding. When temperature decreases the importance of this interaction grows, the occupancy of less occupied position becomes smaller and finally, around 150 K the structure becomes fully ordered.
Graphical Abstract
[One of the water molecule is disordered at room temperature, but when temperature decreases the structure becomes fully ordered around 150 K.]

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Results and Discussion
Benzimidazole compounds, such as omeprazole and lansoprazole, are gastric parietal cell proton pump inhibitors (PPIs), which are widely used for the treatment of acid-related gastric diseases due to their ability to inhibit acid secretion [1]. The title compound (lansoprazole sulphide) is an intermediate for the preparation of lansoprazole. In addition, lansoprazole and its analogs have been reported to have an independent gastroprotective action and selective activity against helicobacter pylori [2]. A comprehensive review on lansoprazole is published [3]. The crystal structures of lansoprazole [4], lansoprazole sulfide salt with chloranilic acid [5] and lansoprazole sulfone [6] have been reported.
Room temperature structure of the title compound—2-[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl]methylthio-1H-benzimidazole hydrate (lansoprazole sulphide hydrate, Scheme 1)—has been briefly reported recently [7]. Here, we report the results of the structural studies in different temperatures; these studies show the ordering of the disordered water molecule.
Molecular Structure
The perspective view of one of the symmetry-independent molecules is shown in Fig. 1. Table 1 lists the relevant geometrical parameters. The diffraction data were collected at different temperatures (vide infra) but as the geometry of the molecule does not change significantly, the description—which will be given for 110 K data—is also valid for other temperatures.
Anisotropic ellipsoid representation of the molecule A together with atom labeling scheme [18]. The ellipsoids are drawn at 50% probability level, hydrogen atoms are depicted as spheres with arbitrary radii. Hydrogen bond is shown as a dashed line
The asymmetric part of the unit cell contains two symmetry-independent molecules of 1 and two water molecules (i.e. Z′ = 2). The molecules are very similar; the normal probability plots [8, 9] for geometrical parameters show only statistical differences between them. The correlation coefficients R 2 between the set of experimental differences and appropriate theoretical values for the normal distribution are around 0.97 for bond lengths and 0.98 for bond angles.
The molecules are approximately planar, the dihedral angles between the planar benzimidazole ring system (planar within 0.027(3) Å for molecule A and 0.010(3) Å for B) and pyridine ring (maximum deviation of 0.009(2) Å for A and 0.007(2) Å for B) are as small as 1.14(10)° and 1.17(7)°. Also the central CSCC bridge is in extended conformation (torsion angles 178.5(2)° and −179.5(2)°). For the least-squares plane calculated for the whole molecule without only fluorine and hydrogen atoms, i.e., through 21 atoms, the maximum deviation is as small as 0.063(3) Å for molecule A and slightly larger, 0.131(3) Å for B– in the latter case the terminal C20 atom is out of much better plane of the rest of the molecule.
The bond lengths and angles are rather typical, with the influence of substituents apparent in the intraannular angles of pyridine ring.
Crystal Packing
The title compound, lansoprazole sulfide, crystallizes as a hydrate, and the water molecule plays an important role in determining the crystal structure. Interestingly, both symmetry independent molecules create the hydrogen-bonded chains on their own, they make hydrogen-bonded chains of molecules along x (molecules A) or along y (molecules B). Hydrogen bond data for the 110 K structure are given in Table 2.
It should be noted, however, that the hydrogen bonded structures created by both symmetry independent molecules are not exactly the same. The analysis might be performed on the subsequent levels on complexity. As the first level one can regard the creation of the chains of alternating 1 and water molecules (Fig. 2a). This motif involves two different hydrogen bonds: N–H···O(water) and O(water)-H···N. Using the graph-set notation [10, 11] this is a second-order chain, C 22 (6). And this motif is common for both symmetry-independent molecules.
Subsequent levels of hydrogen-bonded structure [19] a the C 22 (6) chain of the molecules (A); molecules B make exactly the same motif, b the O–H···N hydrogen bonds connect the neighbouring chains of molecules A, c the O–H···S hydrogen bonds connect chains of B-molecules
Next level of hydrogen-bonded structure is created by the interactions between the chains, and here the situation is different for both quasi-independent chains. First, it should be noted that while the hydrogen bonds in the chains are relatively strong and directional, the interactions between chains are weaker. There is one strong hydrogen bond donor left, namely the second hydrogen of the water molecule. In the structure created by molecules A it definitely points towards the pyridine ring nitrogen (cf. Table 2), and these hydrogen bonds together with the other hydrogen bonds create the centrosymmetric dimers, with the graph set R 22 (18)—cf. Fig. 2b.
For molecules B this O–H bond points towards sulphur atom, and this O–H···S hydrogen bonds in combination with the other N–H···O and O–H···N bonds create also the centrosymmetric dimers but the graph sets which describe the rings are R 22 (12)—Fig. 2c. The O–H···S hydrogen bonds are quite rare, especially in such configuration. In the Cambridge Structural Database [12] we found only 51 structures with similar bonds (CSD version 5.32 of Nov 2010, last update Aug 2011; search criteria: organic molecules, divalent sulfur in non-cyclic environment, H···O distance shorter than the sum of van der Waals radii as defined by the CCDC). In the shortest of this interaction, observed in the structure of 4,4′-sulfanediylbis(2-t-butyl-5-methylphenol) [13], the H···S distance is 2.34 Å, and the O···S 3.286 Å. The mean values of these parameters for all 51 examples are 2.75 and 3.44 Å, respectively. The interaction observed in 1 fits well within this population.
These different interactions might be connected with the observed disorder of the water molecule from B-system; one might speculate that O–H···S hydrogen bonds are weaker and therefore allow for different orientation of the water molecules, providing there is space enough. In fact, the less-occupied water molecule does not take part in any intermolecular interaction, the distance from potential acceptors is too large (cf. Table 2). When temperature falls, the importance of the O–H···S hydrogen bond grows and the water molecules start to order.
Some secondary, but still directional and specific interactions can be also identified within the dimers. In both cases there are relatively short and directional C–H···S interactions (cf. Table 3), also in both cases there is significant stacking between the planar π-electron systems. For the molecules A the distance between the centroids of benzoimidazole and phenyl systems is 3.783 Å, which gives the distance between the planes of ca. 3.46 Å with typical slip of 1.52 Å; in the case of B the distance between the centroids is shorter – 3.606 Å, but the shift is relatively small (0.58 Å) which results in larger interplanar separation of 3.560 Å. For molecules A there are also two C–H···π contacts, which can be regarded as weak hydrogen bonds. Interestingly, there are no such contacts for molecules B.
Structure Change with Lowering Temperature
The unit cell parameters decrease rather uniformly with temperature (cf. Fig. 3). That might suggest the equivalence of different intermolecular interactions that change similarly in all directions. In total, unit cell parameters decrease by ca. 1% when comparing room temperature with 110 K, and the unit cell volume by ca. 3%.
However it turned out that these simple changes are accompanied by the interesting process of ordering of one of the water molecules. The results of data collections in the different modes of temperature change suggest that these changes are fully reversible: this was checked by collecting the data on crystal cooled down to 100 K and then warmed back to room temperature.
At room temperature one (and only one of two symmetry-independent) water molecule is disordered (Fig. 4a); the ratio of the site occupancy factors were reported as 0.79:0.21 in [7], and we determined it as 0.760(17):0.240(17); these values might be regarded as consistent taking into account that they are dependent on the refinement details.
The comparison of the molecules B a at room temperature with disordered water molecule, and b at 110 K without the disorder [18]
We have applied the following procedure for the refinement at different temperatures: we have been using the room-temperature structure as the starting model. For the disordered fragment the s.o.f. (with constraint of adding up to unity) and one common isotropic displacement parameter were initially refined. Then the refined value of s.o.f. was fixed and the individual isotropic and subsequently anisotropic displacement parameters were refined. The anisotropic model was applied for the water molecule with larger s.o.f. in every case, and for the other water molecule only at the room temperature. For smaller occupancies the isotropic model was deemed sufficient in all other cases.
The results show the decrease of the occupancy of less-occupied position when the temperature was lowering (cf. Fig. 5), at 110 K the occupancy became 1 (Fig. 4b), and the disorder vanished. It might be noted that the quadratic fit shown in Fig. 5 is the approximate only and does not suggest any particular mechanism of changes. Two additional quick data collections at 100(1) and 90(1) K confirmed the lack of disorder also at these temperatures. The attempts of collecting the data at higher temperatures were also performed but it turned out that the crystal started to decompose just above the room temperature.
Conclusions
In the room temperature crystal structure of lansoprazole sulfide one of the symmetry-independent water molecules is disordered, although it is involved in important hydrogen bond interactions. When temperature decreases the occupancy of higher occupied position increases and the structure is fully ordered at ca. 150 K. This different behaviour of two symmetry-independent water molecules might be related with the different intermolecular interactions they are involved in: the ordered water molecule in O–H···N while the disordered one in O–H···S.
Experimental
The title compound was prepared with the procedure reported in the literature [14]. Crystals suitable for X-ray diffraction were grown from slow evaporation of ethyl acetate solution.
X-ray diffraction data were collected at various temperatures in the range 110–300 K on three different agilent diffractometers (cf. Table 1 for details). The temperature was controlled by an Oxford Instruments Cryosystems cooling device. The data were corrected for Lorentz-polarization effects as well as for absorption [15]. Accurate unit-cell parameters were determined by a least-squares fit of reflections of highest intensity, chosen from the whole experiment. The structures were solved with SIR92 [16] and refined with the full-matrix least-squares procedure on F 2 by SHELXL97 [17]. Scattering factors incorporated in SHELXL97 were used. The function Σw(∣F o∣2 − F c∣2)2 was minimized, with w−1 = [σ2(F o)2 + A · P2 + B · P], where P = [Max (F 2o , 0) + 2F 2c ]/3. The final values of A and B are listed in Table 1. All non-hydrogen atoms were refined anisotropically, hydrogen atoms were placed in calculated positions and were refined as ‘riding’ on their parent atoms; the Uiso’s of hydrogen atoms were set as 1.2 (1.5 for methyl groups) times the U eq value of the appropriate carrier atom. The details of the refinement procedure related to the disordered water molecule will be described in the “Discussion” section. Relevant crystal data are listed in Table 1, together with refinement details.
Crystallographic data (excluding structure factors) for the structural analysis has been deposited with the Cambridge Crystallographic Data Centre, Nos. CCDC 820703 (110 K), 820704 (150 K), 820705 (200 K), 820706 (250 K) and 820707 (295 K). Copies of this information may be obtained free of charge from: The Director, CCDC, 12 Union Road, Cambridge, CB2 1EZ, UK. Fax: +44(1223)336-033, e-mail:deposit@ccdc.cam.ac.uk, or www.ccdc.cam.ac.uk.
References
Fellemius E, Berglindh T, Sachs G, Elander B, Sjöstrand SE, Wallmark B (1981) Nature 290:159
Iwahi T, Satoh H, Nakao M, Iwasaki T, Yamazaki T, Kubo K, Tamura T, Imada A (1991) Antimicrob Agents Chemother 35:490
Zimmermann AE, Katona BG (1997) Pharmacotherapy 17:308
Vyas K, Sivalkshmidevi A, Om Reddy G (2000) Acta Crystallogr C56:e572
Al-arique QNMH, Jasinski JP, Butcher RJ, Yathirajan HS, Narayana B (2010) Acta Crystallogr E66:o1507
Swamy GYSK, Ravikumar K (2007) J Struct Chem 48:715
Ren G-B, Hong M-H, Zhong J-L, Yi D-X, Xu L-H (2011) Acta Crystallogr E67:o270
Abrahams SC, Keve ET (1971) Acta Crystallogr A27:157
International tables for X-ray crystallography vol IV (1974), p. 293. Kluwer, Dordrecht
Etter MC, MacDonald JC, Bernstein J (1990) Acta Crystallogr B46:256
Bernstein J, Davis RE, Shimoni L, Chang N-L (1995) Angew Chem Int Ed Engl 34:1555
Allen FH (2002) Acta Crystallogr B58:380
Popa LC, Rheingold AL, Beckmann PA (2010) Solid State Nucl Magn Reson 38:31
Kubo K, Oda K, Kaneko T, Satoh H, Nowara A (1990) Chem Pharm Bull 38:2853
Agilent Technologies (2010). CrysAlis PRO
Altomare A, Cascarano G, Giacovazzo C, Guagliardi A (1993) J Appl Crystallogr 26:343
Sheldrick GM (2008) Acta Crystallogr A64:112
Siemens (1989) Stereochemical Workstation Operation manual. Release 3.4. Siemens Analytical X-ray Instruments Inc, Madison
Macrae CF, Bruno IJ, Chisholm JA, Edgington PR, McCabe P, Pidcock E, Rodriguez-Monge L, Taylor R, van de Streek J, Wood PA (2008) J Appl Crystallogr 41:466
Acknowledgments
MTS thanks University of Mysore for research facilities. RJB acknowledges the NSF MRI program (Grant No. CHE-0619278) for funds to purchase the X-ray diffractometer.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Room-temperature crystal structure of this compound has been recently briefly reported: Ren G-B, Hong M-H, Zhong J-L, Yi D-X, Xu L–H (2011) Acta Cryst. E67:o270.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Kubicki, M., Dutkiewicz, G., Jasinski, J.P. et al. Ordering of the Water Molecule in the Crystal Structure of 2-[3-Methyl-4-(2,2,2-Trifluoroethoxy)-2-Pyridinyl]Methylthio-1H-Benzimidazole Hydrate (Lansoprazole Sulphide Hydrate). J Chem Crystallogr 42, 245–250 (2012). https://doi.org/10.1007/s10870-011-0232-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-011-0232-2