Abstract
The α-diimine ligand 1,10-phenanthroline (phen) reacts with the activated cluster 1,2-Os3(CO)10(MeCN)2 to afford the carbonyl-bridged cluster 1,1-Os3(CO)9(μ-CO)(phen), which has been characterized by IR and NMR spectroscopies and X-ray diffraction analysis. Replacement of the 1,5-cyclooctadiene (cod) ligand in 1,1-Os3(CO)10(cod) by phen proceeds sluggishly over a 24 h period, showing less than 5% conversion to 1,1-Os3(CO)9(μ-CO)(phen).
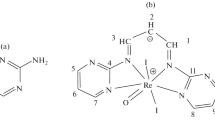
Graphical abstract
α-Diimine Chelation at a Triosmium Cluster: Synthesis and X-ray Structure of 1,1-Os 3 (CO) 9 (μ-CO)(1,10-phen)
Bhaskar Poola, Xiaoping Wang, and Michael G. Richmond
The reaction between the triosmium cluster 1,2-Os3(CO)10(MeCN)2 and 1,10-phenanthroline yields 1,1-Os3(CO)9(μ-CO)(phen) at room temperature. The presence of a bridging carbonyl group in the product cluster has been observed by IR spectroscopy and confirmed by X-ray crystallography.


Similar content being viewed by others
References
(a) Deeming AJ, Peters R, Hursthouse MB, Backer-Dirks JDJ (1982) J Chem Soc Dalton Trans 787; (b) Leadbeater NE, Lewis J, Raithby PR, Ward GN (1999) J Chem Soc Dalton Trans 2511; (c) Nijhoff J, Hartl F, van Outersterp JWM, Stufkens DJ, Calhorda MJ, Veiros LF (1999) J Organomet Chem 573:121; (d) Choi Y-Y, Wong W-T (1999) J Organomet Chem 573:189; (e) van Slageren J, Hartl F, Stufkens DJ, Martino DM, van Willigen H (2000) Coord Chem Rev 208:309; (f) Vergeer FW, Bakker MJ, Kleverlaan CJ, Hartl F, Stufkens DJ (2002) Coord Chem Rev 229:107; (g) Machado RA, Goite MC, Arce AJ, De Sanctis Y, Deeming AJ, D’Ornelas L, Oliveros DA (2005) J Organomet Chem 690:622
For related reports dealing with the reactivity of triosmium clusters containing other nitrogen-based heterocycles, see: (a) Nowroozi-Isfahani T, Musaev DG, Morokuma K, Rosenberg E (2006) Inorg Chem 45:4963; (b) Musaev DG, Nowroozi-Isfahani T, Morokuma K, Abedin J, Rosenberg E, Hardcastle KI (2006) Organometallics 25:203; (c) Mottalib MA, Begum N, Abedin SMT, Akter T, Kabir SE, Miah MA, Rokhsana D, Rosenberg E, Hossain GMG, Hardcastle KI (2005) Organometallics 24:4747; (d) Akther J, Azam KA, Das AR, Hursthouse MB, Kabir SE, Malik KMA, Rosenberg E, Tesmer M, Vahrenkamp H (1999) J Organomet Chem 588:211; (e) Hursthouse MB, Kabir SE, Malik KMA, Tesmer M, Vahrenkamp H (1998) J Organomet Chem 568:133; (f) Zoet R, Jastrzebski JTBH, van Koten G, Mahabliersing T, Vrieze K, Heijdenrijk D, Stam CH (1988) Organometallics 7:2108; (g) Zoet R, van Koten G, Stufkens DJ, Vrieze K, Stam CH (1988) Organometallics 7:2118
Vergeer FW, Kleverlaan CJ, Matousek P, Towrie M, Stufkens DJ, Hartl F (2005) Inorg Chem 44:1319, and references therein
Calhorda MJ, Hunstock E, Veiros LF, Hartl F (2001) Eur J Inorg Chem 223
(a) van Outersterp JWM, Garriga-Oostenbrink MT, Nieuwenhuis HA, Stufkens DJ, Hartl F (1995) Inorg Chem 34:6312; (b) Nijhoff J, Bakker MJ, Hartl F, Stufkens DJ, Fu W-F, Van Eldik R (1998) Inorg Chem 37:661; (c) Bakker MJ, Hartl F, Stufkens DJ, Jina OS, Sun X-Z, George MW (2000) Organometallics 19:4310
Nicholls JN, Vargas MD (1989) Inorg Synth 26:289
Liu Y-C, Yeh W-Y, Lee G-H, Peng S-M (2004) J Organomet Chem 689:1944
Drake SR, Loveday PA (1999) Inorg Synth 28:230
Shriver DF (1969) The manipulation of air-sensitive compounds. McGraw-Hill, New York
APEX2 Version 2.02, Bruker Advanced X-ray Solutions, Inc. Copyright 2005, Madison, WI
SHELXTL Version 6.14, Bruker Advanced X-ray Solutions, Inc. Copyright 2003, Madison, WI
Spek AL (2006) PLATON—a multipurpose crystallographic tool. Utrecht University, Utrecht, The Netherlands
Churchill MR, DeBoer BG (1977) Inorg Chem 16:878
(a) Zoet R, van Koten G, Vrieze K, Duisenberg AJM, Spek AL (1988) Inorg Chim Acta 148:71; (b) Choi Y-Y, Wong W-T (1999) J Chem Soc Dalton Trans 331; (c) Dawoodi Z, Mays MJ, Raithby PR (1981) J Chem Soc Chem Commun 801; (d) Li Y, Wong W-T (2001) J Cluster Sci 12:595; (e) Cabeza JA, del Rio I, Suarez M, Alvarez-Rua C, Garcia-Granda S, Chuang SH, Hwu JR (2003) Eur J Inorg Chem 4159; (f) Li Y, Lin Z-Y, Wong W-T (2001) Eur J Inorg Chem 3163
(a) Churchill MR, Wasserman HJ (1983) J Organomet Chem 248:365; (b) Krause JA, Siriwardane U, Salupo TA, Wermer JR, Knoeppel DW, Shore SG (1993) J Organomet Chem 454:263
A check of mono- and polynuclear phen- and bpy-substituted compounds in the Cambridge Structural Database reveals no significant ground-state differences between closely related phen and bpy analogues
Acknowledgment
Financial support from the Robert A. Welch Foundation (Grant B-1093-MGR) is appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Poola, B., Wang, X. & Richmond, M.G. α-Diimine Chelation at a Triosmium Cluster: Synthesis and X-ray Structure of 1,1-Os3(CO)9(μ-CO)(1,10-phen). J Chem Crystallogr 37, 641–644 (2007). https://doi.org/10.1007/s10870-007-9225-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-007-9225-6