Abstract
People with prediabetes are at risk for type 2 diabetes. They may discount the future delay discounting (DD), and not engage in preventive health behaviors. Episodic future thinking (EFT) can reduce DD when future scenarios are cued, but research is needed to assess long-term effects of EFT and when EFT is not cued. This study tested EFT training compared to control for people with prediabetes enrolled in a 6-month weight loss program on DD, weight, HbA1c, and physical activity. Results showed a reliable EFT effect on reducing DD in cued (p = 0.0035), and uncued DD tasks (p = 0.048), and significant overall changes in weight (p < 0.001), HbA1c (p, 0.001) and physical activity (p = 0.003), but no significant differences in these outcomes by group (p’s > 0.05). Sixty-eight percent of the sample ended below the prediabetes HbA1c range. These results suggest that DD can be modified over extended periods, and the effects of EFT can be observed without EFT cues. However, these data do not suggest that changes in weight, HbA1c or physical activity were due to EFT training. The study was initiated before the COVID-19 pandemic which provided the opportunity to compare differences for people treated in-person or remotely. Analyses showed no differences in DD, weight, HBA1c or physical activity outcomes were observed between in-person and remote treatment, suggesting telehealth is a scalable approach to treating prediabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The overvaluation of immediate rewards and the discounting of future rewards, also known as high delay discounting (DD), is an important factor in affecting how people make decisions. DD is a process related to a wide variety of transdiagnostic problems (Bickel & Mueller, 2009; Bickel et al., 2012). One challenge of a narrow temporal window is encouraging people who discount the future to see the benefit of engaging in preventive health behaviors. Axiomatically, engaging in preventative health requires a focus on the effects of your behavior on future outcomes rather than focusing on immediately reinforcing effects of current behavior. One prototypical example of the relationship between DD and preventive health is prediabetes.
Prediabetes is an intermediate phase in the trajectory from normoglycemia to type 2 diabetes (Nichols et al., 2007; Tabak et al., 2012). People with prediabetes often are high in DD and engage in less healthy diets, less physical activity, and have lower medication adherence (Epstein et al., 2020). Lifestyle change can prevent the transition from prediabetes to type 2 diabetes (Knowler et al., 2002). However, those at risk for diabetes may excessively discount the future (Reach et al., 2011), which, in turn, may lead to a failure to adopt or engage in behaviors that would prevent this transition to type 2 diabetes. The majority of people with prediabetes are obese, and discount the future more than leaner peers (Amlung et al., 2016). Those who are obese and who discount the future eat less healthy diets and are less physically active than leaner peers (Epstein et al., 2020).
We have demonstrated that changes in DD over a year are prospectively related to HbA1c, as those who increased DD the most also showed the most significant increases in HbA1c (Epstein et al., 2019). This relationship was independent of the relationship between weight gain and increases in HbA1c, suggesting that adding an intervention that modifies DD might improve HbA1c beyond weight control, and might also enhance the efficacy of behavioral weight loss (BWL). Devaluing of future rewards may lead individuals with prediabetes to overvalue present rewards and limit the effectiveness of BWL treatments. For this reason, interventions designed to have people focus more on the future may be useful to prevent type 2 diabetes. A proven intervention to reduce DD is episodic future thinking (EFT), which teaches people to think about positive future events (EFT cues) when faced with a situation that involves choosing a small immediate reward versus a larger, but delayed reward. EFT has also been shown to reduce energy intake, and reduce the behavioral economic demand for food (Sze et al., 2017). We have demonstrated in two studies that EFT improves DD in people with prediabetes (Bickel et al., 2020; Stein et al., 2021)., and that EFT improves weight loss (Sze et al., 2015) Also, we have shown that the effect of EFT on DD can improve over time, with repeated revision of episodic future cues, suggesting people learn to create more usable cues and how to use those cues in tempting situations (Mellis et al., 2019).
One important EFT component in experimental lab-based studies is prompting the use of positive, vivid EFT cues when engaged in a DD task. A long-term goal of EFT is to change the default prospective thinking of people when they are making choices, even if they have not been prompted to engage in EFT. At present, no research has shown the effect of EFT training and rehearsal on DD without the explicit prompt to think about EFT cues, suggesting that the goal of modifying default prospective thinking has not been achieved.
Lifestyle programs for diabetes target improving diet and increasing activity, but diet represents the most crucial component for weight loss and glycemic control changes (Knowler et al., 2002). Dietary changes to reduce type 2 diabetes risk may involve reducing energy intake, carbohydrate intake (Wood & Fernandez, 2009), or consuming lower glycemic index carbohydrates, which can stabilize blood glucose levels and improve glycemic control (Ludwig, 2002). This may be challenging since highly palatable, high glycemic foods, such as baked goods and ice cream, and everyday dietary staples including breads, rice, and pastas may be very reinforcing (Epstein et al., 2007). The reinforcing effects of these foods may make it difficult to limit their consumption or to consume other less reinforcing foods. Deprivation of these foods only increases their value (Epstein et al., 2003; Flack et al., 2019; Raynor & Epstein, 2003). Food deprivation focuses attention on the immediate reward of consuming food rather than the accrual of future benefits that can occur with ongoing lifestyle changes. Given that reducing carbohydrate intake can improve glycemic control, (Elhayany et al., 2010; Mayer et al., 2014; Wood & Fernandez, 2009) the BWL program utilized in this study modified diet in a stepwise fashion, focusing on reducing energy intake and then substituting low glycemic index carbohydrates for higher glycemic index carbohydrates, and finally reduced total carbohydrate intake.
The present study was designed to test the effects of EFT on cued and uncued delay discounting, weight, HbA1c and physical activity, and test whether changes in DD mediated effects of EFT on these variables. In addition, this study was implemented during the COVID-19 pandemic, which required the cessation of all in-person meetings and the switch to remote intervention part way through study completion. This provided the opportunity to compare in-person versus remote treatments for episodic future thinking, weight loss and glycemic control in people with prediabetes.
Methods
Participants and procedures
Participants with prediabetes (HbA1c between 5.7 and 6.4% (39–46 mmol/mol) (American Diabetes Association, 2021) were recruited from the Buffalo, New York and Roanoke, Virginia communities. Participants were at least 18 years of age, had no prior or current diagnosis of diabetes, were not pregnant, and were not taking medications that influenced their blood glucose. Nine hundred and thirty three people completed a prescreen internet survey, two hundred and ninety four people were screened in our laboratories, and seventy two began the study. Sixty-four people were randomized to BWL + EFT or BWL + DCI control group. Characteristics of the participants are presented in Table 1. Procedures followed guidelines for the ethical conduct of human research outlined by the National Institute of Health and with approval of the University at Buffalo and the Virginia Tech Institutional Review Boards.
Design
All participants received the same BWL starting in weeks one through six. At week seven, participants were randomized across three study cohorts to one of two groups, BWL + EFT or BWL + Daily Check In (DCI). An approximately equal number of people were randomized to experimental and control groups at each site, with approximately 10 people randomized per cohort at each site to each group. Participants attended assessments at 0, 3, and 6 months, including measures of height, weight, cued and uncued DD and physical activity.
Procedure
Participants were recruited from primary care and endocrinology practices, advertisements on Facebook and in newspapers, community flyers, direct mailings of postcards, emails sent to staff at each site, and databases of prior research participants maintained by each study site. Interested people completed online screening questionnaires which included questionnaires about alcohol and drug use, eating disorder symptomology, and those who met the initial criteria were scheduled for an in-person laboratory visit that involved a collection of HbA1c values, measures of height and weight, the five-item adjusting delay DD task (Koffarnus & Bickel, 2014), and general lifestyle questions to screen for eligibility and were asked to wear an ActiGraph WGT3X-BT accelerometer (ActiGraph, Pensacola, FL, USA) over the week following the baseline visit. After completing baseline, participants were scheduled to begin treatment. Within each cohort at each site, treatment and control groups were run concurrently.
Common characteristics of BWL included group meetings held weekly for the first 8 weeks, then every other week through week 12, and monthly from months 4 through 6. For the first six weeks of treatment, participants met with the same group on a week-to-week basis and learned essential components of the BWL intervention regarding diet, physical activity and other lifestyle changes. Before the week 7 treatment session, participants were randomized into the BWL + EFT or DCI groups. The assignment was stratified by participant baseline HbA1c and weight loss from weeks 1 to 6 to equate groups on important dependent measures.
Participants were weighed during each treatment meeting, attended an interactive group meeting, and an individual meeting with their case manager. In weeks when group meetings were not taking place, participants completed case management via phone call with the case manager. They were also provided a treatment manual with lessons matched to group discussion topics. The treatment included the Traffic Light Diet and Activity program adapted for diabetes and behavioral tools, including self-monitoring, creating physical alternatives to food, substitution, stimulus control, preplanning, menu preparation, goal setting, and self-reinforcement.
The dietary approach used the Traffic Light Diet as an organizing principle to increase healthy foods while reducing the intake of less healthy foods. Foods are categorized as RED, YELLOW, or GREEN foods based on their energy density and nutrient density to foster the most nutrients for the fewest calories. Foods are categorized into nine categories, vegetables, starchy vegetables, fruit, grains, dairy, protein, fats/oils/sweet/others, soups, condiments/dressings/other ingredients. Within each category, foods are categorized as GREEN, YELLOW or RED based on their nutrient density for that type of food. The only GREEN foods were low energy density, low calorie non-starchy vegetables, which were considered GO foods, so they will have a satiating effect as people reduce their energy intake. YELLOW foods were within 20 cal of the USDA energy values established for that category of food, and RED foods were above 20 cal greater per serving of the USDA energy values established for that category of food. According to macronutrients, many foods were labeled according to glycemic index where such information could be found from glycemic index testing or by their relation to USDA tested foods. Foods were labeled VERY LOW if their glycemic index was 35 or lower, LOW if the glycemic index was 36 to 55, MODERATE if the glycemic index was 56 to 69, and HIGH if the glycemic index was 70 + (Atkinson et al., 2008; Foster-Powell & Miller, 1995). The Traffic Light Activity Plan was also used which rates activities are RED, YELLOW or GREEN based on their MET (metabolic equivalent) values, with sedentary activities (< 3 METS) rated as RED, moderate to vigorous activities (4–5 METS) rated as YELLOW, and activities greater than 6 METS rated as GREEN activities. To assist participants in identifying foods and activities conforming to program goals, participants were also provided a Traffic Light Cookbook and a Food and Activity Reference Guide, the latter of which was also made into a searchable online database. These gave the traffic light color and glycemic indices of foods or recipes and the traffic light color of activities. A glycemic index cheat sheet displaying low or very low glycemic index foods with 15 or fewer grams of carbs was created and provided to participants at week 16.
As part of treatment, all participants were provided calorie goals (1200–1500 cal/day), RED and GREEN food goals (two or fewer servings of RED foods and five or more servings of GREEN foods daily), and activity goals (150 min of moderate-intensity physical activity/week or 75 min vigorous physical activity/week). The diet was implemented to reduce energy intake for weeks 0–16, monitoring glycemic index, and substituting low glycemic index foods starting in week 2. Then from week 16 on, we added a focus on reducing carbohydrates to 100 or fewer grams per day. All participants were given an account on the food and activity tracking app, MyFitnessPal, and were instructed to record all foods consumed, physical activities performed, and record their weight at least once a week. These accounts were accessible to research staff and used by case managers to monitor participant engagement in plans and progress towards case management goals.
EFT implementation
Before EFT began, participants were emailed a link to a survey developed in our previous research (Bickel et al., 2020; Stein et al., 2021) to guide them through the generation of EFT cues at 7 different future time points matching those used in the DD task (1 month, 3 months, 6 months, 1 year, 3 years, 5 years, and 10 years). Moreover, they were told to complete the survey before their next treatment session (week 7). Instructions for cue creation were as follows,
“In this task, you will be asked to list and describe future events that you are looking forward to. These events must be vivid and easy for you to imagine.
You will do this for 7 different time periods. These events can be big or small and could be related to your long-term health goals. All that matters is that you are looking forward to them. After describing these events, you will rate each event on several characteristics.
These are events that could really happen or that you have planned.”
Participants were then asked to think about and name an event corresponding to each of the delay periods. For each event, they were told it should be something they are looking forward to, that they can vividly imagine, and that they should start with the words “In about [DELAY], I am…” Examples of good and bad “I am” statements were shown to illustrate the desired format. They then gave the event's anticipated date and the “I am” statement (event tag) for each time period. After creating each event tag, participants were then asked to elaborate in detail about each event. They were given the following instruction.
“Imagine yourself time traveling to the future to experience your event. Describe your event as though you are experiencing everything right now. Include in your description: Who is with you? What are you doing? Where are you? How are you feeling? Also:
Use as much detail as possible. (For example, rather than "I am at a coffee shop," say "I am sitting on the outdoor terrace at a coffee shop downtown.")
Try to focus on the positive parts of your experience.
In the box below, type your event description (about 3–4 sentences). Make sure your description is positive and has vivid details! Remember to use "I am" statements and include: Where you are, who you are with, what you are doing, how you are feeling.”
After detailing each event, they were asked to rate it for enjoyment, excitement, importance, and vividness. If a participant had not completed the survey by the day of their visit, the case manager would call to assist with cue creation. If cues were lacking in detail, the case manager would call to have the participant elaborate on their event(s) to attain those details. Finally, if a participant did not complete the survey before arriving for their week 7 appointment, they were guided through cue creation by a research staff member before case management as has been done previously (Bickel et al., 2020; Stein et al., 2021). From week 7 on, EFT participants' treatment materials included instruction on utilizing EFT cues to employ the behavioral tools taught. Case management included pre-planning and rehearsal of cue use to meet specific goals to overcome anticipated challenges. Every four weeks after that, participants were asked to re-generate their 1 and 3 month cues to keep them current and were given the option of regenerating any other EFT cues.
EFT was implemented using the Mobile Audio Manager and Response Tracker (MAMRT) app (Sze et al., 2015) that provided the opportunity to have EFT cues provided to participants on their smartphones and answer questions about what decisions they were using cues to assist them in making. After generation, all EFT cues were immediately imported into MAMRT for use as part of the intervention. Through MAMRT, participants were sent email and text message reminders to log in to practice envisioning their EFT cues. Each time a participant logged in to MAMRT, they would first be presented with the event tag from three of their seven cues chosen randomly and were asked to click on at least one to view the full cue. They were asked to vividly imagine that cue, then to click to another page and answer two questions. The first question asked them to choose from a list which healthy decisions (if any) they were planning to make by envisioning the cue, and the second was an open-ended prompt for a brief description of that upcoming challenge. Responses were reviewed by case managers and used to help prompt discussion of cue effectiveness and timing of MAMRT prompts. Participants were prompted to log into MAMRT 3 times a day (morning, afternoon, evening) for two weeks, then prompts reduced to twice a day for six weeks, and finally to once a day for weeks 15 onward. Participants were also provided EFT cues printed on individual 3″ × 5″ cards bearing a picture of a traffic light on the reverse and one card bearing a list of all the program's behavioral tools. These cards were attached to a key ring and provided to participants for their use outside the digital environment.
DCI implementation
For the DCI group, treatment materials from randomization onward continued with teaching behavioral tools without the mention of EFT. To minimize prospection in case management, the focus was shifted from pre-planning to reviewing recent past challenges and what one could have done differently. To equate groups on exposure to MAMRT, DCI participants were prompted to log in with the same frequency as EFT participants and at the same times of day, but were displayed a blank page in lieu of EFT cues. After clicking on the next page, they were asked the same questions about what decisions they were attempting to make and describe a challenge; however, they were instructed to report their most recently experienced past decision and challenge instead of prospecting on upcoming challenges. These answers were also used in case management and allowed the case managers to ask for details about specific challenges participants might otherwise fail to mention. DCI participants were also given cue cards to equate for exposure to study material outside of MAMRT; however, instead of bearing EFT cues, cards had the name of an individual behavioral tool printed on them (ex. Stimulus Control) in addition to one card with all tools listed.
COVID-19 procedures
As the COVID-19 pandemic interrupted normal procedures, several adaptations were made to allow continued treatment in a remote environment. The intervention was delivered in three cohorts. The first cohort was delivered totally in-person (prior to COVID-19), with no study activities interrupted by COVID-19. The second cohort was treated ½ in person and ½ remotely (immediately prior to and during the initial outbreak), and the third was treated remotely. This situation provided the unique opportunity to compare in-person versus remote treatments for weight loss and glycemic control in people with prediabetes. The same treatment protocol was delivered for all three cohorts. Research suggests that diabetes interventions can be implemented remotely (Su et al., 2016). The potential for remote intervention and remote measurement can increase the intervention's scalability by not requiring people to attend in-person clinic visits to receive the treatment.
All study activities intended to occur in the lab were transitioned to occurring over ZOOM video conference using separate breakout rooms for participants to meet with their case manager and have their weight taken. During treatment sessions, participants met in a separate breakout room with a team member who would instruct each participant how to take their weight in the manner it is done in the clinical setting on a digital scale mailed to participants. After weighing themselves, participants were instructed to show the digital display to the team member recording the weight to verify the virtual weight. Then participants completed both the larger group meeting and individual case management over Zoom. In group sessions, presentation materials were shared via screen share, and copies of materials that would have been provided in-person were emailed to participants to use during the session. As an additional safety measure during the pandemic, short questionnaires addressing feelings of stress, loneliness, and perceived risk of catching COVID-19 were created and conducted every four weeks before or after case management in another separate breakout room.
For participants unable to have a laboratory assessment (baseline, 12-weeks, or 24-weeks) due to COVID-19, remote assessments were also conducted via ZOOM utilizing the screen-share function to allow participants to enter responses when necessary. For these visits, at-home HbA1c test kits were mailed to participants, and blind assessors instructed participants on how to test their A1c during the virtual assessments. Accelerometers were also mailed to participants with self-addressed and stamped return envelopes so that they could begin with physical activity monitoring after completing virtual assessments and return the device to the lab.
Measurement
Demographics were measured using a standardized questionnaire. Weight, height, and DD were measured by assessors blinded to the treatment group. Weight was measured using a Tanita (Hong Kong, China) digital scale. Height was measured using a SECA (Chino, California) stadiometer. HbA1c was measured in the laboratory using the Alere Afinion AS100 HbA1c measuring system, while the remote assessments were collected using the validated (Bode et al., 2007; Hirst et al., 2017) A1CNow + ® system (PTS Diagnostics, Sunnyvale, CA). Extensive instructions and access to assessment personnel were used to collect the outcome weight, HbA1c, and DD measures. During the weekly remote meetings, weights were measured using a validated Beautural digital body weight scale (Shenzhen 1Byone Technology Co., Ltd. Shenzhen, China) sent to participants.
Objective measures of physical activity were obtained for 7 days using the Actigraph WGT3X-BT accelerometer (AG; ActiGraph, Pensacola, FL) at baseline, and at months 3 and 6. The Actigraph WGT3X-BT accelerometer is a well validated measure of physical activity (Crouter et al., 2010; Sasaki et al., 2011). Participants were asked to wear the monitor on their non-dominant side for one week, snug to the hip during waking and non-swimming/bathing/showering. Participants recorded the time the device was worn in a wear time log. Data were collected at a rate of 30 Hz and were filtered first for non-wear time based on 90 consecutive minutes of non-wear (Choi et al., 2011) and then filtered based on participants’ wear time diaries. Participants wore the accelerometer an average of 91.0 ± 18.62 h. The dependent measures were average vector magnitude counts per minute which measure motion and percent of time engaged in moderate to vigorous physical activity (MVPA, > 3.00 METS and was calculated by the Freedson Adult VM3 algorithm (Sasaki et al., 2011).
DD was assessed using an adjusting amount task (Du et al., 2002) where choices were presented between a larger, delayed amount of money ($100) and a smaller, immediate amount. The smaller, immediate amount began at $50 on the first trial and was adjusted following each trial (up or down), depending on the participants’ choice. Specifically, after each trial, a choice for the larger reward increased the smaller, immediate amount; whereas a choice for the smaller, adjusting amount decreased this option. The size of each adjustment (up or down) began at $25 and decreased by half at each subsequent trial ($12.50, $6.25, etc.). The final adjusted amount of the smaller, immediate option after the final, sixth trial served as the indifference point, which provides an index of the subjective value of the delayed reward. For example, if a participant is indifferent between $70 now and $100 after a delay, this indicates that the delayed reward has been devalued by 30%. This titration process was repeated at each of seven delays to the larger reward, in randomized order (1 month, 3 months, 6 months, 1 year, 3 years, 5 years, 10 years).
To calculate discount rates, we fit individual participants’ discounting curves (indifference points as a function of delay) using Mazur’s (Mazur, 1987) hyperbolic discounting model,
where V is discounted value, A is reward amount, D is delay, and k is a free parameter that indexes the rate of discounting. (Stein et al., 2017) Higher values of k indicate more rapid devaluation of the delayed reward and hence greater impulsivity. For the adjusting-amount task, k was derived by fitting individual participants’ indifference amounts across delays using nonlinear regression. Values of k were positively skewed and were thus natural log-transformed before analysis.
At baseline and the week following the 24-week assessment, DD was measured without instructions to imagine the episodic future cues, while at randomization, the 12-week and 24-week assessments DD was measured with instructions to imagine EFT cues, or, for the DCI group, with blank pages added where EFT participants would see cues. The instructions for the cued DD read,
“You will now be presented with a series of choices relating to money. During each of these choices, you will imagine a specific event that you created. You will imagine the event in as much detail as possible. These questions are hypothetical, but please choose your answer as if you will receive the money in the time frame selected. Please pay close attention to the amount and time frame of each option, and choose accordingly. There are no right or wrong answers in this task. Please take your time.”
Then, before each delay, EFT participants were instructed to slowly read aloud and imagine their corresponding event displayed in its entirety. When making each choice, they were asked which option they would rather receive when imagining their event. For these questions, a one-sentence tag of the event naming the time period and the participant's action was displayed. For DCI participants, instructions were identical, except they were not told any event would be displayed or to imagine an event before each delay or when making each choice. Before each delay, a blank screen appeared in lieu of an EFT cue.
Adherence to use of MMART logins to present EFT or DCI cues was monitored by MMART and presented in terms of number of logins presented over the study, and percent of logins used in relationship to the number that were prompted.
Analytic plan
Initial analyses involved chi-square tests and t-tests to compare baseline values of key variables across groups for both outcomes. The primary analyses to compare between-group changes in weight, HbA1c and DD were mixed model ANCOVA that used an unstructured covariance structure and included random effects of participant with Group (BWL + EFT, BWL + DCI) and Cohort (In person, 50% in person/50% remote, 100% remote) as the between variables, weeks as the repeated measures, and site (UB/VT) as a covariate. Linear contrasts were used to compare changes over time. Study cohort was added as a between-subject variable to evaluate whether treatment effectiveness differs by in-person versus remote status. Mixed model ANCOVA analyses were used for intention to treat and included all randomized participants in the final analysis. All data are presented mean ± SE.
Relationships between changes in weight, HbA1c, and DD were calculated using zero-order correlations. The percentage of people who transitioned from prediabetes to non-prediabetes by the group was analyzed using Chi-Square tests.
Power for this study is based on our observed correlation of changes in DD predicting changes in HbA1c, r = 0.33. A correlation of 0.33 requires 70 subjects to show a significant effect at the power of 0.80 and alpha of 0.05. Also, we have recently demonstrated EFT to reduce DD in a sample of people with prediabetes (Bickel et al., 2020) which required 74 subjects to achieve 0.80 power at 0.05 alpha. We recruited 91% of the estimated sample size.
Results
Characteristics of the sample are shown in Table 1. None of the variables were different between groups. As shown in Fig. 1, analysis of cued DD showed a significant differential improvement in DD by group over the six months of the study (F(2,57) = 6.26, p = 0.0035), as well as a significant overall change by time (F(2,57) = 27.49, p < 0.0001). No significant differences in DD were observed by group for the three COVID conditions (F(4,57) = 0.36, p = 0.84). Linear contrasts showed significant differences between group from 0–12 weeks (t(57) = 2.07, p < 0.001), 12 to 24 weeks (t(57) = 2.08, p = 0.042), and 0–24 weeks (t(57) = 2.40, p = 0.0195). The average DD change at 12 weeks and 24 weeks across groups and cohorts was − 1.95 ± 0.30 and − 1.99 ± 0.27, respectively.
Delay discounting for EFT versus DCI control groups at 0, 12 and 24 weeks for cued tasks (A), and values at 0, 12 and 24 weeks for each cohort for the EFT (B) and DCI (C) groups (mean ± SEM) for each cohort. Cohort 1 was seen in-person, cohort 2 both in person and remotely, and cohort 3 was seen remotely
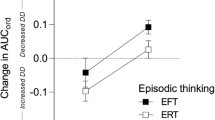
Analysis of uncued DD also showed a significant differential improvement by group (Fig. 2) over the 6 months of the study (F(1,58) = 4.09, p = 0.048), as well as a significant overall change by time (F(1,58) = 46.46, p < 0.0001). No significant differences in uncued DD were observed by COVID cohort for the three COVID conditions (F(2,58) = 1.34, p = 0.27). The average uncued DD change at 24 weeks across groups and cohorts was − 1.68 ± 1.99.
Delay discounting for EFT versus DCI control groups at 0 and 24 weeks for uncued tasks (A), and values for each cohort at 0 and 24 weeks for each cohort for the EFT (B) and DCI (C) groups (mean ± SEM) for each cohort. Cohort 1 was seen in-person, cohort 2 both in person and remotely, and cohort 3 was seen remotely
While between group differences were observed for both cued and uncued DD tasks, the DCI group did show significant overall improvement overtime. The DCI group showed significant improvement in cued DD from 0–12 weeks (t(30) = 2.44, p = 0.02) and 0–24 (t(30) = 3.78, p < 0.001) weeks, as well as significant improvement in uncued DD from 0–25 weeks (t(30) = 4.12, p < 0.001).
Analysis of adherence showed significant differences between the groups, with participants in EFT engaging in more logins to present EFT or DCI cues (218.0 ± 24.5 vs 137.0 ± 23.9) and did so more often than prompted (115.4 ± 13.9 vs 72.5 ± 12.7%; F(1,57) = 5.62, p = 0.02).
ITT analysis of weight showed a significant reduction in weight over the 6 months of the study (F(2,57) = 92.46, p < 0.001, but no differences by group (F(2,57) = 0.17, p = 0.85), or by cohort (F(4,57) = 0.91, p = 0.45). Contrasts comparing changes over time showed significant weight decreases from 0–12 weeks (t(57) = 13.48, p < 0.001), 12 to 24 weeks (t(57) = 8.18, p < 0.001), and 0–24 weeks (t(57) = 13.01, p < 0.001). The average weight change at 12 weeks and 24 weeks across groups and cohorts was − 6.9 kg ± 0.5 and − 9.9 ± 0.76, respectively.
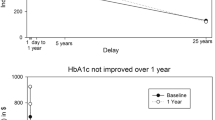
Analysis of HbA1c changes also showed a significant reduction over time (F(2, 57) = 41.06, p < 0.001), but no differences by treatment condition (F(2,57) = 0.23, p = 0.79), or by cohort (F(4,57) = 0.85, p = 0.50). Linear contrasts showed significant differences from 0–12 weeks (t(57) = 6.14 p < 0.001), 12–24 weeks (t(57) = 3.10, p = 0.003, and 0–24 weeks (t(57) = 8.92, p < 0.001). The average HbA1c change at 12 weeks and 24 weeks across groups and cohorts was − 0.24 ± 0.32 and − 0.36 ± 0.33, respectively. By 12 weeks, 68% of participants were below 5.7% HbA1c, and by 24 weeks, 68% of the participants were below 5.7%, indicating resolution of prediabetes, with no significant differences by group (X2(1) = 1.68, p = 0.20). Figure 3 shows the means and standard errors for the weight (left graph) and HbA1c values (right graph) for the three cohorts.
Analysis of accelerometer vector magnitude counts per minute showed a significant improvement over time (F(2, 56) = 12.28, p < 0.001), but no differences by group (F(2,56) = 1.06, p = 0.35), or by cohort (F(4,56) = 0.84, p = 0.51). Linear contrasts showed significant improvements from 0–12 weeks (t(56) = 4.84 p < 0.001), and 0–24 weeks (t(56) = 2.95, p = 0.0046), but not between 12–24 weeks (t(56) = 1.00, p = 0.32). The average increase in vector magnitude at 12 weeks and 24 weeks across groups and cohorts was 68.41 ± 14.14 and 51.05 ± 17.29, respectively. Percent time in MVPA showed a significant improvement over time (F(2, 56) = 9.47, p = 0.003), but no differences by treatment condition (F(2,56) = 1.08, p = 0.35), or by cohort (F(4,56) = 0.63, p = 0.64). Linear contrasts showed significant improvements from 0–12 weeks (t(56) = 4.24, p < 0.001), and 0–24 weeks (t(56) = 2.48, p = 0.016), but not between 12–24 weeks (t(56) = 1.27,, p = 0.21). The average MVPA percent increase at 12 weeks and 24 weeks across groups and cohorts was 1.03 ± 0.24 and 0.66 ± 0.27, respectively. The percent increases are equivalent to increases of 14.8 and 9.5 min per day of additional MVPA. Figure 4 shows the means and standard errors for the vector magnitude counts (left graph) and % MVPA values (right graph) for the three cohorts.
Correlational analysis showed that 0–24 week weight change was related to HbA1c change (r = 0.38, p = 0.003), and HbA1c changes were related to baseline HbA1c (r = − 0.32, p 0.012). In addition, baseline HbA1c was related to baseline DD (r = 0.32, p = 0.01). Adherence to EFT/DCI was related to weight change (r = − 0.33, p = 0.01). None of the other correlations were significant.
Discussion
Effects of EFT on DD.
The results show that EFT can reduce DD over 6 months compared to a daily check-in control, and that the EFT effect generalized from cued to uncued tasks, suggesting that participant’s default way of thinking or evaluation of the future may have changed. While the finding that EFT-modified DD is not new, this is the first demonstration that prolonged EFT training can alter DD even if people are not concurrently cued at the time of DD assessment to engage in using the EFT cues. This is of paramount importance for clinical EFT interventions and suggests that EFT cues may not be needed after sufficient training for many basic temporal decisions, while cues can be made available for use for more challenging temporal decisions such as those that involve consumption of high-glycemic, high energy dense foods. This is an important finding that should be replicated and extended to different EFT training protocols.
Participants in the EFT group logged into the mobile EFT app 15% more than just when prompted, suggesting they did take advantage of the app to use in situations that may have evolved outside of the prompt schedule. In addition, the number of EFT cues used was related to weight loss, as those who used the mobile app more showed greater weight loss. Surprisingly, no relationship was observed between using the mobile app and changes in cued or uncued delay discounting. This suggests that optimal EFT dose to reduce delay discounting when prompted, or the optimal dose to modify default decision-making is unknown, as more does not necessarily result in greater decreases in delay discounting. Participants may have already been above the threshold needed to shift default decision making.
Small but significant changes were observed for the DCI control group in both the cued and uncued DD tasks. This may have been a function of one of two factors. First, there may be a learning effect where people improve with repeated testing, as people improved from 0 to 12 weeks, with further significant improvement from 12 to 24 weeks. Second, BWL can improve DD without any training in EFT (Ross et al., 2020). BWL may include treatment components that foster a more prospective mindset, leading to reductions in DD. Prominent among these components is preplanning and menu planning, which require people to learn how to plan for the future. Since these are standard components of treatment that are engaged in regularly, even for people not trained in EFT, they may help reduce DD.
Weight loss and glycemic control
The results replicate many previous studies that show weight loss can improve glycemic control. The dietary approach, which included reductions in calories and a gradual shift in the glycemic index of foods and the total number of carbohydrates, produced large decreases in weight of almost 10 kg, and clinically significant reduction in HbA1c of − 0.36%. In addition to the reductions in HbA1c, 68% of randomized participants converted from prediabetes to euglycemia after treatment. Of course, all behavioral weight loss programs are not the same. These changes in HbA1c are larger than those observed during the Diabetes Prevention Program, which observed differences in HbA1c of 0.1% at 6 months (Maruthur et al., 2013). Previous studies have shown that including carbohydrate reductions in a calorie-reduced diet can improve glycemic control beyond weight loss, even if the weight losses are similar (Elhayany et al., 2010; Mayer et al., 2014; Sainsbury et al., 2018). This study could not isolate the differential effect of lowering the glycemic index of food versus reducing the amount of carbohydrates. This question should be addressed by future research as reducing food with a high glycemic index may be easier than reducing carbohydrate intake.
Some of the behavioral weight loss program's effectiveness is likely due to the choice of low glycemic foods and a reduction in carbohydrate intake to a goal of no more than 100 g per day. Previous research has shown that low carbohydrate or low glycemic index diets can amplify weight loss effects beyond energy restriction. The Traffic Light Diet used for obesity treatment was adapted for prediabetes, which categorized foods into the RED, YELLOW, and GREEN colors based nutrient density and energy density, supplemented with information about the glycemic index of foods. The Traffic Light represents an easy-to-follow program that reduces much of the decision making on what to eat to the idea of eating more GREEN foods (non-starchy vegetables) and fewer RED foods (high energy dense and/or high glycemic). Future studies might compare a simplified Traffic Light Diet to traditional diets for people with prediabetes or type 2 diabetes in energy restriction but who are socially or educationally disadvantaged and live in scarcity conditions. These factors can impact decision making and could impact the extent to which diets are understood and implemented.
Changes in DD as a mediator of improvements in weight and glycemic control
Adding EFT to a standardized behavioral weight loss program was not shown to improve weight control or HbA1c during this 6-month investigation. A likely reason was that the intensive behavioral weight loss program that both groups received—comprising diet and physical activity education, interactive group meetings, individualized case management, and self-monitoring of diet, physical activity, and weight—was very efficacious, thereby creating a ceiling effect. As such, this efficacious treatment blunted the additive impact of EFT beyond the other aspects of treatment. The weight loss of almost 10 kg at 6 months represents slightly larger changes than observed in the Diabetes Prevention Program at 6 months, considered the gold standard in weight loss for people with prediabetes (Delahanty et al., 2013). Also, the HbA1c changes of − 0.36% HbA1c were greater than the 0.1% observed in the Diabetes Prevention Program at 6 months (Maruthur et al., 2013). The observation that prediabetes resolved in 68% of people suggests the general BWL treatment may have put a ceiling on the potential for greater changes, or may require a more intensive form of the EFT component to observe further improvement.
In addition, EFT may be more beneficial for people during weight loss maintenance, or the prevention of weight gain, than in the weight loss process. Weight control interventions are typically challenged over the long-term by weight plateau or rebound weight gain, which EFT may modulate since EFT aims to focus people on long-term goals. An important motivation for eating in people with obesity is the reinforcing value of food, which can be increased after dieting and food restriction (Epstein et al., 2007), shifting focus more on immediate rewards associated with eating, and less on long-term temporal goals. Testing EFT at various treatment points would be interesting to see if it can improve long-term weight control and reduce increases in food reinforcement that may occur with energy restriction (Flack et al., 2019).
Cross-sectional studies have shown that baseline DD is related to baseline HBA1c in this and previous studies (Epstein et al., 2020), and changes in DD were prospectively related to changes in HbA1c over a year (Epstein et al., 2019). Studies have shown EFT can modify eating behavior in the laboratory (Daniel et al., 2013) in a food court setting (O'Neill et al., 2016) and food shopping behavior (Hollis-Hansen et al., 2019, 2020). Improvements in DD observed in the laboratory are also observed when tested in the natural environment (Bickel et al., 2020). Despite this research on the potential of EFT for modifying DD as a mechanism for behavior change, experimental manipulations of DD in this study did not result in improvements in every day decision making that resulted in greater weight loss or improvements in glycemic control. While this may be due to a ceiling effect on weight or HbA1c change due to the intensive behavioral weight loss program, we cannot disregard the possibility that reducing discounting of the future is not a relevant mediator for improving weight loss or glycemic control in people with prediabetes.
Adaptations to COVID
A potentially important finding from this study was the absence of differences in weight, HbA1c, or DD outcomes by cohort, representing the successful implementation of the interventions totally in person, one-half in person, and one-half remotely, and fully remote during the COVID-19 pandemic. This difference in implementation was necessary due to the safety procedures implemented by both universities following the escalation of the COVID-19 pandemic, which shut down in-person meetings. However, this was not an experimental manipulation, which has to be considered in evaluating these differences. For example, different participants may volunteer to be part of a clinical study during a pandemic instead of during non-pandemic times.
Despite these considerations, the data suggest that the weight loss and EFT treatments can be implemented remotely without any loss of treatment effectiveness. A growing literature on remote interventions and remote delivery of the Diabetes Prevention Program showed no differences in weight loss between those seen in person or remotely (Vadheim et al., 2017). Still, the changes (5.5 kg) were less than observed in the current study. An interesting goal of future studies would be to experimentally manipulate the delivery of the behavioral weight loss program used in this study and evaluate the cost–benefit of remote versus in-person treatments. Considerable differences in the cost to participants would be expected, particularly related to time saved in travel, as would cost for treatment delivery.
To our knowledge, there are no previous studies comparing treatment delivery during non-pandemic versus pandemic conditions. We have previously shown that EFT can reduce DD even under scarcity conditions that usually increase DD (Bickel et al., 2020), but this is the first study to demonstrate the efficacy of EFT during real-world stress conditions. This has tremendous implications for treatment for people who are attempting to change their behaviors in the context of stressful or anxiety-provoking situations. The demonstration that uncued DD is modified is evidence that a person’s default way of thinking can be modified even during personal and social stress.
In summary, this study shows that the effects of EFT can be observed over 6 months, and they generalize to delay discounting tasks in which people are not cued for EFT. The amount and type of training that can result in a shift in default thinking is important given the transdiagnostic role of DD across a wide variety of diseases. The results also showed that a Traffic Light low carbohydrate, low glycemic index diet adapted for prediabetes and type 2 diabetes can result in significant reductions in weight and improvements in glycemic control, and that this intervention, in combination with EFT, can be delivered using remote technology.
References
American Diabetes Association. (2021). 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2021. Diabetes Care, 44, S15–S33. https://doi.org/10.2337/dc21-S002
Amlung, M., Petker, T., Jackson, J., Balodis, I., & MacKillop, J. (2016). Steep discounting of delayed monetary and food rewards in obesity: A meta-analysis. Psychological Medicine, 46, 2423–2434. https://doi.org/10.1017/S0033291716000866
Atkinson, F. S., Foster-Powell, K., & Brand-Miller, J. C. (2008). International tables of glycemic index and glycemic load values: 2008. Diabetes Care, 31, 2281–2283. https://doi.org/10.2337/dc08-1239
Bickel, W. K., Jarmolowicz, D. P., Mueller, E. T., Koffarnus, M. N., & Gatchalian, K. M. (2012). Excessive discounting of delayed reinforcers as a trans-disease process contributing to addiction and other disease-related vulnerabilities: Emerging evidence. Pharmacology & Therapeutics, 134, 287–297. https://doi.org/10.1016/j.pharmthera.2012.02.004
Bickel, W. K., & Mueller, E. T. (2009). Toward the study of Trans-Disease Processes: A novel approach with special reference to the study of co-morbidity. Journal of Dual Diagnosis, 5, 131–138. https://doi.org/10.1080/15504260902869147
Bickel, W. K., Stein, J. S., Paluch, R. A., Mellis, A. M., Athamneh, L. N., Quattrin, T., Greenawald, M. H., Bree, K. A., Gatchalian, K. M., Mastrandrea, L. D., & Epstein, L. H. (2020). Does episodic future thinking repair immediacy bias at home and in the laboratory in patients with prediabetes? Psychosomatic Medicine, 82, 699–707. https://doi.org/10.1097/PSY.0000000000000841
Bode, B. W., Irvin, B. R., Pierce, J. A., Allen, M., & Clark, A. L. (2007). Advances in hemoglobin A1C point of care technology. Journal of Diabetes Science and Technology, 1, 405–411.
Choi, L., Liu, Z. W., Matthews, C. E., & Buchowski, M. S. (2011). Validation of accelerometer wear and nonwear time classification algorithm. Medicine and Science in Sports and Exercise, 43, 357–364. https://doi.org/10.1249/MSS.0b013e3181ed61a3
Crouter, S. E., Kuffel, E., Haas, J. D., Frongillo, E. A., & Bassett, D. R. (2010). Refined two-regression model for the ActiGraph accelerometer. Medicine and Science in Sports and Exercise, 42, 1029–1037. https://doi.org/10.1249/MSS.0b013e3181c37458
Daniel, T. O., Stanton, C. M., & Epstein, L. H. (2013). The future is now: Reducing impulsivity and energy intake using episodic future thinking. Psychological Science, 24, 2339–2342. https://doi.org/10.1177/0956797613488780
Delahanty, L. M., Peyrot, M., Shrader, P. J., Williamson, D. A., Meigs, J. B., Nathan, D. M., & Group, D. P. P. R. (2013). Pretreatment, psychological, and behavioral predictors of weight outcomes among lifestyle intervention participants in the Diabetes Prevention Program (DPP). Diabetes Care, 36, 34–40. https://doi.org/10.2337/dc12-0733
Du, W. J., Green, L., & Myerson, J. (2002). Cross-cultural comparisons of discounting delayed and probabilistic rewards. Psychological Record, 52, 479–492.
Elhayany, A., Lustman, A., Abel, R., Attal-Singer, J., & Vinker, S. (2010). A low carbohydrate Mediterranean diet improves cardiovascular risk factors and diabetes control among overweight patients with type 2 diabetes mellitus: A 1-year prospective randomized intervention study. Diabetes, Obesity and Metabolism, 12, 204–209. https://doi.org/10.1111/j.1463-1326.2009.01151.x
Epstein, L. H., Leddy, J. J., Temple, J. L., & Faith, M. S. (2007). Food reinforcement and eating: A multilevel analysis. Psychological Bulletin, 133, 884–906. https://doi.org/10.1037/0033-2909.133.5.884
Epstein, L. H., Paluch, R. A., Stein, J. S., Mellis, A. M., Quattrin, T., Mastrandrea, L. D., Bree, K. A., Greenawald, M. H., & Bickel, W. K. (2019). Role of delay discounting in predicting change in HBA1c for individuals with prediabetes. Journal of Behavioral Medicine, 42, 851–859. https://doi.org/10.1007/s10865-019-00026-3
Epstein, L. H., Paluch, R. A., Stein, J. S., Quattrin, T., Mastrandrea, L. D., Bree, K. A., Sze, Y. Y., Greenawald, M. H., Biondolillo, M. J., & Bickel, W. K. (2020). Delay discounting, glycemic regulation and health behaviors in adults with prediabetes. Behavioral Medicine. https://doi.org/10.1080/08964289.2020.1712581
Epstein, L. H., Truesdale, R., Wojcik, A., Paluch, R. A., & Raynor, H. A. (2003). Effects of deprivation on hedonics and reinforcing value of food. Physiology and Behavior, 78, 221–227.
Flack, K. D., Ufholz, K., Casperson, S., Jahns, L., Johnson, L., & Roemmich, J. N. (2019). Decreasing the consumption of foods with sugar Increases their reinforcing value: A potential barrier for dietary behavior change. Journal of the Academy of Nutrition and Dietetics, 119, 1099–1108. https://doi.org/10.1016/j.jand.2018.12.016
Foster-Powell, K., & Miller, J. B. (1995). International tables of glycemic index. American Journal of Clinical Nutrition, 62, 871S-890S. https://doi.org/10.1093/ajcn/62.4.871S
Hirst, J. A., McLellan, J. H., Price, C. P., English, E., Feakins, B. G., Stevens, R. J., & Farmer, A. J. (2017). Performance of point-of-care HbA1c test devices: Implications for use in clinical practice—A systematic review and meta-analysis. Clinical Chemistry and Laboratory Medicine, 55, 167–180. https://doi.org/10.1515/cclm-2016-0303
Hollis-Hansen, K., Seidman, J., O’Donnell, S., & Epstein, L. H. (2019). Episodic future thinking and grocery shopping online. Appetite, 133, 1–9. https://doi.org/10.1016/j.appet.2018.10.019
Hollis-Hansen, K., Seidman, J., O’Donnell, S., Wedderburn, A., Stanar, S., Brande, S., & Epstein, L. H. (2020). An ecological momentary episodic future thinking intervention on mother’s weekly food purchases. Health Psychology, 39, 159–167. https://doi.org/10.1037/hea0000817
Knowler, W. C., Barrett-Connor, E., Fowler, S. E., Hamman, R. F., Lachin, J. M., Walker, E. A., & Nathan, D. M. (2002). Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New England Journal of Medicine, 346, 393–403.
Koffarnus, M. N., & Bickel, W. K. (2014). A 5-trial adjusting delay discounting task: Accurate discount rates in less than one minute. Experimental and Clinical Psychopharmacology, 22, 222–228. https://doi.org/10.1037/a0035973
Ludwig, D. S. (2002). The glycemic index—Physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. Journal of the American Medical Association, 287, 2414–2423. https://doi.org/10.1001/jama.287.18.2414
Maruthur, N. M., Ma, Y., Delahanty, L. M., Nelson, J. A., Aroda, V., White, N. H., Marrero, D., Brancati, F. L., Clark, J. M., Diabetes Prevention Program Research, G. (2013). Early response to preventive strategies in the Diabetes Prevention Program. Journal of General Internal Medicine, 28, 1629-1636. https://doi.org/10.1007/s11606-013-2548-4
Mayer, S. B., Jeffreys, A. S., Olsen, M. K., McDuffie, J. R., Feinglos, M. N., & Yancy, W. S., Jr. (2014). Two diets with different haemoglobin A1c and antiglycaemic medication effects despite similar weight loss in type 2 diabetes. Diabetes Obesity and Metabolism, 16, 90–93. https://doi.org/10.1111/dom.12191
Mazur, J. E. (1987). An adjusting procedure for studying delayed reinforcement. In M. L. Commons, J. E. Mazur, J. A. Nevin, & H. Rachlin (Eds.), Quantitative analyses of behavior: The effect of delay and of intervening events on reinforcement value (Vol. 5, pp. 55–73). Lawrence Erlbaum Associates, Inc.
Mellis, A. M., Snider, S. E., Deshpande, H. U., LaConte, S. M., & Bickel, W. K. (2019). Practicing prospection promotes patience: Repeated episodic future thinking cumulatively reduces delay discounting. Drug and Alcohol Dependence, 204, 107507. https://doi.org/10.1016/j.drugalcdep.2019.06.010
Nichols, G. A., Hillier, T. A., & Brown, J. B. (2007). Progression from newly acquired impaired fasting glucose to type 2 diabetes. Diabetes Care, 30, 228–233. https://doi.org/10.2337/dc06-1392
O’Neill, J., Daniel, T. O., & Epstein, L. H. (2016). Episodic future thinking reduces eating in a food court. Eating Behaviors, 20, 9–13. https://doi.org/10.1016/j.eatbeh.2015.10.002
Raynor, H. A., & Epstein, L. H. (2003). The relative-reinforcing value of food under differing levels of food deprivation and restriction. Appetite, 40, 15–24.
Reach, G., Michault, A., Bihan, H., Paulino, C., Cohen, R., & Le Clesiau, H. (2011). Patients’ impatience is an independent determinant of poor diabetes control. Diabetes & Metabolism, 37, 497–504. https://doi.org/10.1016/j.diabet.2011.03.004
Ross, K. M., Eastman, A., Ugwoaba, U. A., Demos, K. E., Lillis, J., & Wing, R. R. (2020). Food reward sensitivity, impulsivity, and weight change during and after a 3-month weight loss program. PLoS ONE, 15, e0243530. https://doi.org/10.1371/journal.pone.0243530
Sainsbury, E., Kizirian, N. V., Partridge, S. R., Gill, T., Colagiuri, S., & Gibson, A. A. (2018). Effect of dietary carbohydrate restriction on glycemic control in adults with diabetes: A systematic review and meta-analysis. Diabetes Research and Clinical Practice, 139, 239–252. https://doi.org/10.1016/j.diabres.2018.02.026
Sasaki, J. E., John, D., & Freedson, P. S. (2011). Validation and comparison of ActiGraph activity monitors. Journal of Science and Medicine in Sport, 14, 411–416. https://doi.org/10.1016/j.jsams.2011.04.003
Stein, J. S., Craft, W. H., Paluch, R. A., Gatchalian, K. M., Greenawald, M. H., Quattrin, T., Mastrandrea, L. D., Epstein, L. H., & Bickel, W. K. (2021). Bleak present, bright future: II. Combined effects of episodic future thinking and scarcity on delay discounting in adults at risk for type 2 diabetes. Journal of Behavioral Medicine, 44, 222–230. https://doi.org/10.1007/s10865-020-00178-7
Stein, J. S., Sze, Y. Y., Athamneh, L., Koffarnus, M. N., Epstein, L. H., & Bickel, W. K. (2017). Think fast: Rapid assessment of the effects of episodic future thinking on delay discounting in overweight/obese participants. Journal of Behavioral Medicine, 40, 832–838. https://doi.org/10.1007/s10865-017-9857-8
Su, D., Zhou, J., Kelley, M. S., Michaud, T. L., Siahpush, M., Kim, J., Wilson, F., Stimpson, J. P., & Pagan, J. A. (2016). Does telemedicine improve treatment outcomes for diabetes? A meta-analysis of results from 55 randomized controlled trials. Diabetes Research and Clinical Practice, 116, 136–448. https://doi.org/10.1016/j.diabres.2016.04.019
Sze, Y. Y., Daniel, T. O., Kilanowski, C. K., Collins, R. L., & Epstein, L. H. (2015). Web-based and mobile delivery of an Episodic Future Thinking intervention for overweight and obese families: a feasibility study. JMIR mHealth and uHealth, 16, e97.
Sze, Y. Y., Stein, J. S., Bickel, W. K., Paluch, R. A., & Epstein, L. H. (2017). Bleak present, bright future: Online episodic future thinking, Scarcity, delay discounting, and food demand. Clinical Psychological Science, 5, 683–697. https://doi.org/10.1177/2167702617696511
Tabak, A. G., Herder, C., Rathmann, W., Brunner, E. J., & Kivimaki, M. (2012). Prediabetes: A high-risk state for diabetes development. Lancet, 379, 2279–2290. https://doi.org/10.1016/S0140-6736(12)60283-9
Vadheim, L. M., Patch, K., Brokaw, S. M., Carpenedo, D., Butcher, M. K., Helgerson, S. D., & Harwell, T. S. (2017). Telehealth delivery of the diabetes prevention program to rural communities. Translational Behavioral Medicine, 7, 286–291. https://doi.org/10.1007/s13142-017-0496-y
Wood, R. J., & Fernandez, M. L. (2009). Carbohydrate-restricted versus low-glycemic-index diets for the treatment of insulin resistance and metabolic syndrome. Nutrition Reviews, 67, 179–183. https://doi.org/10.1111/j.1753-4887.2009.00186.x
Acknowledgements
This research was funded in part by the National Institutes of Health (NIH) Science of Behavior Change Common Fund Program through an award administered by the National Institute of Diabetes and Digestive and Kidney Diseases (1UH2DK109543), awarded to Drs. Epstein and Bickel. Appreciation is expressed to Dr. Katelyn Carr who assisted with data prep for this revision.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Bickel is a consultant or has equity in HealthSim LLC, NotifiUs LLC, Sober Grid Inc., DxRx, Prophase LLC, Teva Branded Pharmaceuticals, General Genetic Corporation. Leonard H. Epstein, Rocco A. Paluch, Mathew J. Biondolillo, Jeff S. Stein, Teresa Quattrin, Lucy D. Mastrandrea, Kirstin Gatchalian and Mark H. Greenawald do not declare any conflict of interest with respect to the authorship or publication of this article.
Ethical approval
All procedures performed were in accordance with the ethical standards of the institution and with the 1964 Helsinki declaration and its later amendment of comparable ethical standards.
Human and animal rights
Procedures followed guidelines for the ethical conduct of human research outlined by the National Institute of Health and with approval of the University at Buffalo and the Virginia Tech Institutional Review Boards.
Informed consent
Informed consent was obtained from all participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Epstein, L.H., Paluch, R.A., Biondolillo, M.J. et al. Effects of 6-month episodic future thinking training on delay discounting, weight loss and HbA1c changes in individuals with prediabetes. J Behav Med 45, 227–239 (2022). https://doi.org/10.1007/s10865-021-00278-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10865-021-00278-y