Abstract
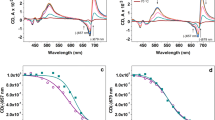
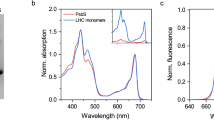
Isoprene emission protects plants from a variety of abiotic stresses. It has been hypothesized to do so by partitioning into cellular membranes, particularly the thylakoid membrane. At sufficiently high concentrations, this partitioning may alter the physical properties of membranes. As much as several per cent of carbon taken up in photosynthesis is re-emitted as isoprene but the concentration of isoprene in the thylakoid membrane of rapidly emitting plants has seldom been considered. In this study, the intramembrane concentration of isoprene in phosphatidylcholine liposomes equilibrated to a physiologically relevant gas phase concentration of 20 μL L−1 isoprene was less than predicted by ab initio calculations based on the octanol-water partitioning coefficient of isoprene while the concentration in thylakoid membranes was more. However, the concentration in both systems was roughly two orders of magnitude lower than previously assumed. High concentrations of isoprene (2000 μL L−1 gas phase) failed to alter the viscosity of phosphatidylcholine liposomes as measured with perylene, a molecular probe of membrane structure. These results strongly suggest that the physiological concentration of isoprene within the leaves of highly emitting plants is too low to affect the dynamics of thylakoid membrane acyl lipids. It is speculated that isoprene may bind to and modulate the dynamics of thylakoid embedded proteins.




Similar content being viewed by others
References
Affek HP, Yakir D (2002) Protection by isoprene against singlet oxygen in leaves. Plant Physiol 129:269–277. doi: 10.1104/pp.010909
Antunes-Madeira M, Madeira V (1985) Partition of lindane in synthetic and native membranes. Biochim Biophys Acta 820:165–172. doi: 10.1016/0005-2736(85)90109-9
Antunes-Madeira M, Madeira V (1986) Partition of DDT in synthetic and native membranes. Biochim Biophys Acta 861:159–164. doi: 10.1016/0005-2736(86)90414-1
Antunes-Madeira M, Madeira V (1987) Partition of malathion in synthetic and native membranes. Biochim Biophys Acta 901:61–66. doi: 10.1016/0005-2736(87)90256-2
Behnke K, Loivamäki M, Zimmer I, Rennenberg H, Schnitzler J-P, Louis S (2010) Isoprene emission protects photosynthesis in sunfleck exposed Grey Poplar. Photosynth Res 104:5–17. doi: 10.1007/s11120-010-9528-x
Brannigan G, LeBard DN, Hénin J, Eckenhoff RG, Klein ML (2010) Multiple binding sites for the general anesthetic isoflurane identified in the nicotinic acetylcholine receptor transmembrane domain. Proc Natl Acad Sci U S A 107:14122–14127. doi: 10.1073/pnas.1008534107
Brunori M, Vallone B, Cutruzzolà F, Travaglini-Allocatelli C, Berendzen J, Chu K, Sweet RM, Schlichting I (2000) The role of cavities in protein dynamics: crystal structure of a photolytic intermediate of a mutant myoglobin. Proc Natl Acad Sci U S A 97:2058–2063.
Cantor RS (1997) The lateral pressure profile in membranes: a physical mechanism of general anesthesia. Biochemistry (Mosc) 36:2339–2344.
Chapman DJ, Barber J (1986) Analysis of plastoquinone-9 levels in appressed and non-appressed thylakoid membrane regions. Biochim Biophys Acta Bioenerg 850:170–172.
Copolovici LO, Niinemets Ü (2005) Temperature dependencies of Henry’s law constants and octanol/water partition coefficients for key plant volatile monoterpenoids. Chemosphere 61:1390–1400. doi: 10.1016/j.chemosphere.2005.05.003
Demmig B, Winter K, Krüger A, Czygan F-C (1987) Photoinhibition and zeaxanthin formation in intact leaves: a possible role of the xanthophyll cycle in the dissipation of excess light energy. Plant Physiol 84:218–224.
Droppa M, Masojidek J, Horváth G (1990) Changes of the polypeptide composition in thylakoid membranes during differentiation. Z Für Naturforschung C 45:253–257.
Eckenhoff MF, Eckenhoff RG (1998) Quantitative autoradiography of halothane binding in rat brain. J Pharmacol Exp Ther 285:371–376.
Eckenhoff RG, Johansson JS (1997) Molecular interactions between inhaled anesthetics and proteins. Pharmacol Rev 49:343–368.
Eriksson A, Baase W, Zhang X, Heinz D, Blaber M, Baldwin E, Matthews B (1992a) Response of a protein-structure to cavity-creating mutations and its relation to the hydrophobic effect. Science 255:178–183. doi: 10.1126/science.1553543
Eriksson AE, Baase WA, Wozniak JA, Matthews BW (1992b) A cavity-containing mutant of T4 lysozyme is stabilized by buried benzene. Nature 355:371–373. doi: 10.1038/355371a0
Frimer AA (1985) Singlet O2. CRC Press, Boca Raton, Fla.
Ghirardo A, Wright LP, Bi Z, Rosenkranz M, Pulido P, Rodríguez-Concepción M, Niinemets Ü, Brüggemann N, Gershenzon J, Schnitzler J-P (2014) Metabolic flux analysis of plastidic isoprenoid biosynthesis in poplar leaves emitting and nonemitting isoprene. Plant Physiol 165:37–51. doi: 10.1104/pp.114.236018
González-Cabanelas D, Wright LP, Paetz C, Onkokesung N, Gershenzon J, Rodríguez-Concepción M, Phillips MA (2015) The diversion of 2-C-methyl-d-erythritol-2,4-cyclodiphosphate from the 2-C-methyl-d-erythritol 4-phosphate pathway to hemiterpene glycosides mediates stress responses in Arabidopsis thaliana. Plant J 82:122–137. doi: 10.1111/tpj.12798
Hall M, Mishra Y, Schroder WP (2011) Preparation of stroma, thylakoid membrane, and lumen fractions from Arabidopsis thaliana chloroplasts for proteomic analysis. In: Jarvis RP (ed) Chloroplast Research in Arabidopsis: Methods and Protocols, Vol II. Humana Press Inc, Totowa, pp 207–222
Hubbard SJ, Argos P (1994) Cavities and packing at protein interfaces. Protein Sci 3:2194–2206.
Hubbard SJ, Gross K-H, Argos P (1994) Intramolecular cavities in globular proteins. Protein Eng 7:613–626. doi: 10.1093/protein/7.5.613
Janero D, Barrnett R (1981) Cellular and thylakoid-membrane phospholipids of Chlamydomonas reinhardtii 137 + . J Lipid Res 22:1126–1130.
Jardine KJ, Monson RK, Abrell L, Saleska SR, Arneth A, Jardine A, Ishida FY, Serrano AMY, Artaxo P, Karl T, Fares S, Goldstein A, Loreto F, Huxman T (2012) Within-plant isoprene oxidation confirmed by direct emissions of oxidation products methyl vinyl ketone and methacrolein. Glob Change Biol 18:973–984. doi: 10.1111/j.1365-2486.2011.02610.x
Jiang Y, Blanchard GJ (1994) Rotational diffusion dynamics of perylene in n-alkanes. Observation of a solvent length-dependent change of boundary condition J Phys Chem 98:6436–6440.
Kirchhoff H, Mukherjee U, Galla H-J (2002) Molecular architecture of the thylakoid membrane: lipid diffusion space for plastoquinone. Biochemistry (Mosc) 41:4872–4882. doi: 10.1021/bi011650y
Koan MM, Blanchard GJ (2006) Gauging the effect of impurities on lipid bilayer phase transition temperature. J Phys Chem B 110:16584–16590. doi: 10.1021/jp061506s
Kóta Z, Horváth LI, Droppa M, Horváth G, Farkas T, Páli T (2002) Protein assembly and heat stability in developing thylakoid membranes during greening. Proc Natl Acad Sci U S A 99:12149–12154. doi: 10.1073/pnas.192463899
Lemmon MA, Engelman DM (1994) Specificity and promiscuity in membrane helix interactions. Q Rev Biophys 27:157–218. doi: 10.1017/S0033583500004522
Li Z, Ratliff EA, Sharkey TD (2011) Effect of temperature on postillumination isoprene emission in oak and poplar. Plant Physiol 155:1037–1046. doi: 10.1104/pp.110.167551
Logan BA, Anchordoquy TJ, Monson RK, Pan RS (1999) The effect of isoprene on the properties of spinach thylakoids and phosphatidylcholine liposomes. Plant Biol 1:602–606. doi: 10.1111/j.1438-8677.1999.tb00269.x
Loreto F, Velikova V (2001) Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127:1781–1787. doi: 10.1104/pp.010497
Loreto F, Mannozzi M, Maris C, Nascetti P, Ferranti F, Pasqualini S (2001) Ozone quenching properties of isoprene and its antioxidant role in leaves. Plant Physiol 126:993–1000.
Luxnat M, Galla H (1986) Partition of chlorpromazine into lipid bilayer-membranes - the effect of membrane-structure and composition. Biochim Biophys Acta 856:274–282. doi: 10.1016/0005-2736(86)90037-4
Marqusee J, Dill KA (1986) Solute partitioning into chain molecule interphases - monolayers, bilayer-membranes, and micelles. J Chem Phys 85:434–444. doi: 10.1063/1.451621
Mène-Saffrané L, Dubugnon L, Chetelat A, Stolz S, Gouhier-Darimont C, Farmer EE (2008) Nonenzymatic oxidation of trienoic fatty acids contributes to reactive oxygen species management in Arabidopsis. J Biol Chem 284:1702–1708. doi: 10.1074/jbc.M807114200
Miller KW (1985) The nature of the site of general anesthesia. Int Rev Neurobiol 27:1–61.
Miller K, Hammond L, Porter E (1977) The solubility of hydrocarbon gases in lipid bilayers. Chem Phys Lipids 20:229–241. doi: 10.1016/0009-3084(77)90039-1
Palmeira CM, Oliveira CR (1992) Partitioning and membrane disordering effects of dopamine antagonists: Influence of lipid peroxidation, temperature, and drug concentration. Arch Biochem Biophys 295:161–171. doi: 10.1016/0003-9861(92)90502-N
Pang K-Y, Chang T-L, Miller KW (1979) On the coupling between anesthetic induced membrane fluidization and cation permeability in lipid vesicles. Mol Pharmacol 15:729–738.
Pillman HA, Blanchard GJ (2010) Effects of ethanol on the organization of phosphocholine lipid bilayers. J Phys Chem B 114:3840–3846. doi: 10.1021/jp910897t
Pollastri S, Tsonev T, Loreto F (2014) Isoprene improves photochemical efficiency and enhances heat dissipation in plants at physiological temperatures. J Exp Bot 65:1565–1570. doi: 10.1093/jxb/eru033
Rashin AA, Iofin M, Honig B (1986) Internal cavities and buried waters in globular proteins. Biochemistry (Mosc) 25:3619–3625. doi: 10.1021/bi00360a021
Seigneurin-Berny D, Salvi D, Joyard J, Rolland N (2001) Purification of intact chloroplasts from Arabidopsis and spinach leaves by isopycnic centrifugation. Current Protocols in Cell Biology. John Wiley & Sons, Inc.,
Sharkey TD, Singsaas E (1995) Why plants emit isoprene. Nature 374:769–769. doi: 10.1038/374769a0
Sharkey TD, Yeh S (2001) Isoprene emission from plants. Ann Rev Plant Physiol Plant Mol Biol 52: 407–436.
Sharkey TD, Chen X, Yeh S (2001) Isoprene increases thermotolerance of fosmidomycin-fed leaves. Plant Physiol 125:2001–2006.
Simon S, Gutknecht J (1980) Solubility of carbon-dioxide in lipid bilayer-membranes and organic-solvents. Biochim Biophys Acta 596:352–358. doi: 10.1016/0005-2736(80)90122-4
Singsaas EL, Lerdau M, Winter K, Sharkey TD (1997) Isoprene increases thermotolerance of isoprene-emitting species. Plant Physiol 115:1413–1420.
Siwko ME, Marrink SJ, de Vries AH, Kozubek A, Schoot Uiterkamp AJM, Mark AE (2007) Does isoprene protect plant membranes from thermal shock? A molecular dynamics study. Biochim Biophys Acta - Biomembr 1768:198–206. doi: 10.1016/j.bbamem.2006.09.023
Smith R, Porter E, Miller K (1981) The solubility of anesthetic-gases in lipid bilayers. Biochim Biophys Acta 645:327–338. doi: 10.1016/0005-2736(81)90204-2
Triantaphylidès C, Havaux M (2009) Singlet oxygen in plants: production, detoxification and signaling. Trends Plant Sci 14:219–228. doi: 10.1016/j.tplants.2009.01.008
Triantaphylidès C, Krischke M, Hoeberichts FA, Ksas B, Gresser G, Havaux M, Van Breusegem F, Mueller MJ (2008) Singlet oxygen is the major reactive oxygen species involved in photooxidative damage to plants. Plant Physiol 148:960–968. doi: 10.1104/pp.108.125690
Van Eerden FJ, de Jong DH, de Vries AH, Wassenaar TA, Marrink SJ (2015) Characterization of thylakoid lipid membranes from cyanobacteria and higher plants by molecular dynamics simulations. Biochim Biophys Acta Biomembr 1848:1319–1330. doi: 10.1016/j.bbamem.2015.02.025
Velikova VB, Pinelli P, Pasqualini S, Reale L, Ferranti F, Loreto F (2005) Isoprene decreases the concentration of nitric oxide in leaves exposed to elevated ozone: Rapid report. New Phytol 166:419–426. doi: 10.1111/j.1469-8137.2005.01409.x
Velikova VB, Várkonyi Z, Szabó M, Maslenkova L, Nogues I, Kovács L, Peeva V, Busheva M, Garab G, Sharkey TD, Loreto F (2011) Increased thermostability of thylakoid membranes in isoprene-emitting leaves probed with three biophysical techniques. Plant Physiol 157:905–916. doi: 10.1104/pp.111.182519
Velikova VB, Sharkey TD, Loreto F (2012) Stabilization of thylakoid membranes in isoprene-emitting plants reduces formation of reactive oxygen species. Plant Signal Behav 7:139–141. doi: 10.4161/psb.7.1.18521
Velikova VB, Ghirardo A, Vanzo E, Merl J, Hauck SM, Schnitzler J-P (2014) Genetic manipulation of isoprene emissions in poplar plants remodels the chloroplast proteome. J Proteome Res 13:2005–2018. doi: 10.1021/pr401124z
Velikova V, Müller C, Ghirardo A, Rock TM, Aichler M, Walch A, Schmitt-Kopplin P, Schnitzler J-P (2015) Knocking Down of Isoprene Emission Modifies the Lipid Matrix of Thylakoid Membranes and Influences the Chloroplast Ultrastructure in Poplar. Plant Physiol 168:859–870. doi: 10.1104/pp.15.00612
Vickers CE, Gershenzon J, Lerdau MT, Loreto F (2009a) A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat Chem Biol 5:283–291. doi: 10.1038/nchembio.158
Vickers CE, Possell M, Cojocariu CI, Velikova VB, Laothawornkitkul J, Ryan A, Mullineaux PM, Nicholas Hewitt C (2009b) Isoprene synthesis protects transgenic tobacco plants from oxidative stress. Plant Cell Environ 32:520–531. doi: 10.1111/j.1365-3040.2009.01946.x
Wintermans JFGM, de Mots A (1965) Spectrophotometric characteristics of chlorophylls a and b and their phenophytins in ethanol. Biochim Biophys Acta - Biophys Photosynth 109:448–453. doi: 10.1016/0926–6585(65)90170–6
Xiao Y, Savchenko T, Baidoo EEK, Chehab WE, Hayden DM, Tolstikov V, Corwin JA, Kliebenstein DJ, Keasling JD, Dehesh K (2012) Retrograde signaling by the plastidial metabolite MEcPP regulates expression of nuclear stress-response genes. Cell 149:1525–1535. doi: 10.1016/j.cell.2012.04.038
Acknowledgments
The authors thank Shelagh Ferguson-Miller and Michael Feig for helpful discussions; Scott Bankroff for assistance in the design and manufacture of the equilibration and gas stripping apparatus; Professor John Ohlrogge and Dr. Dylan Kosma for helpful discussions regarding FAME. This material is based upon work supported by the National Science Foundation under Grant No. 0950574. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. Partial salary support for TDS comes from Michigan State University AgBioResearch.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 178 kb)
Appendixes
Appendixes
Appendix 1
Anisotropy was calculated using the induced optical anisotropy function:
where I∥(t) and I⊥(t) are the fluorescence emission intensities parallel and perpendicular to the vertically polarized excitation pulse, respectively. The resulting anisotropy decay curves were fitted using Origin 8 software. Fit parameters for individual acquisitions were pooled for statistical analysis. For the transition dipole moment that was pumped and probed (S1-S0), the model for perylene reorientation as a prolate rotor is:
The model for perylene reorientation as an oblate rotor is:
In the model equations, Dx and Dz are the Cartesian components of the rotational diffusion constant (Jiang and Blanchard 1994). Data fitting to the prolate rotor model was by a single exponential decay function.
Data fitting to the oblate rotor model was by a constrained double exponential decay function,
where constraints were “A” between 10−4 and 0.2, “τ1” between 20 and 5000, “τ 2” between 1 and 1000, and “τ 1” between 1.33 and 20 multiples of “τ 2”. Adjusted R-squared was used to compare the fit of the single and double exponential decay models to the data. For the single exponential decays that characterized the data, the Debye-Stokes-Einstein equation was used to calculate viscosity (Koan and Blanchard 2006).
τ OR is the anisotropy decay time constant, η is viscosity, V is the hydrodynamic volume of perylene, f is a solvent-solute frictional interaction coefficient, kB is the Boltzmann constant, T is the absolute temperature, and S is a shape factor to account for the non-spherical shape of perylene. The hydrodynamic volume of perylene is 225 Å3, and the shape factor is 0.7. The translational diffusion coefficient, DT, was estimated using the Stokes-Einstein equation,
where R is the radius of perylene. R was calculated from the hydrodynamic volume of perylene, 225 Å3, by assuming a spherical shape, yielding R = 3.77 Å.
Appendix 2
The concentration of isoprene in DMPC bilayers equilibrated to a gaseous standard state of 20 μL L−1 was estimated from the Henry’s constant (HI) and KOW (KOW,I) of isoprene. The system was assumed to be at 25 °C and 1 atm pressure. The Henry’s constant of a substance is the ratio of its concentration in water to its gas phase partial pressure, in a system at equilibrium. The KOW of a substance is the ratio of its molar concentration in octanol to its molar concentration in water, in a system at equilibrium. At 25 °C, HI = 7780 Pa m3 mol−1 and KOW,I = 263 (Copolovici and Niinemets 2005). The area per lipid (aDMPC) and bilayer thickness (DP-P,DMPC) of a DMPC bilayer were taken to be 0.63 nm2 and 3.4 nm, respectively (Siwko et al. 2007).
The partial pressure of isoprene (PI) was taken as the product of the total atmospheric pressure (PT) and the mole fraction of isoprene in air (XI):
The molar concentration of isoprene in water (MI,W) was calculated from PI and HI:
Multiplying the molar concentration of isoprene in water by the KOW of isoprene yielded the molar concentration of isoprene in octanol (or in a bilayer) (MI,O):
The molecular volume of DMPC in a DMPC bilayer (VDMPC) was calculated from aDMPC and DP-P,DMPC:
The inverse of VDMPC yielded the molar concentration of DMPC in a DMPC bilayer (MDMPC):
Dividing the molarity of isoprene from Eq. 10 by the molarity of DMPC from Eq. 12 yielded the mole fraction of isoprene in a DMPC bilayer (XI,DMPC):
Calculations were performed similarly for thylakoids, except that the area per lipid and membrane thickness were taken to be 0.66 nm2 and 2.9 nm, respectively (van Eerden et al. 2015).
Rights and permissions
About this article
Cite this article
Harvey, C.M., Li, Z., Tjellström, H. et al. Concentration of isoprene in artificial and thylakoid membranes. J Bioenerg Biomembr 47, 419–429 (2015). https://doi.org/10.1007/s10863-015-9625-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-015-9625-9