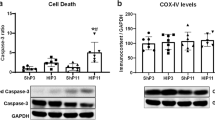
Abstract
Neonatal anoxia at the time of birth can lead to mitochondrial dysfunction and further neurodevelopmental abnormalities. The present study investigated the mitochondrial bioenergetics and associated sensorimotor changes in the anoxic neonatal rats. Rat pups after 30 h to birth (2 days) were subjected to anoxia of two episodes (10 min in each) at a time interval of 24 h by passing 100 % N2 into an enclosed chamber. Brain mitochondrial respiration was measured using clark type oxygen electrode. A significant decrease in brain respiratory control ratio (RCR; State III/IV respiration) at all-time points, complex I (24 h) and complex II (30 min, 6 and 24 h) enzyme activities indicated loss of mitochondrial integrity and function A significant increase in levels of nitric oxide was observed after second anoxic episode at all-time points. A significant change in sensorimotor activity in terms of increased reflex latency was observed 24 h after second episode in this model, which is an indication of loss of subcortical maturation. All the above changes were observed after second but not after the first anoxic exposure. Therefore, this anoxic model shows significant changes in mitochondrial bioenergetics, nitric oxide levels and sensorimotor effects after second episode of anoxia. This model may be helpful to evaluate mitochondrial targeted pharmacological intervention for the treatment of anoxia.




Similar content being viewed by others
References
Benz R, McLaughlin S (1983) The molecular mechanism of action of the proton ionophore FCCP (carbonylcyanide p-trifluoromethoxyphenylhydrazone). Biophys J 41:381–398
Berman SB, Hastings TG (1999) Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria. J Neurochem 73:1127–1137
Bolaños JP, Almeida A, Medina JM (1998) Nitric oxide mediates brain mitochondrial damage during perinatal anoxia. Brain Res 787:117–122
Borutaite V, Toleikis A, Brown GC (2013) In the eye of the storm: mitochondrial damage during heart and brain ischaemia. FEBS J 280:4999–5014
Fan L-W, Lin S, Pang Y, Lei M, Zhang F, Rhodes PG, Cai Z (2005) Hypoxia-ischemia induced neurological dysfunction and brain injury in the neonatal rat. Behav Brain Res 165:80–90
Gilmer LK, Ansari MA, Roberts KN, Scheff SW (2010) Age-related changes in mitochondrial respiration and oxidative damage in the cerebral cortex of the Fischer 344 rat. Mech Ageing Dev 131:133–143
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15 N]nitrate in biological fluids. Anal Biochem 126:131–138
Herrera-Marschitz M (2014) Perinatal asphyxia: CNS development and deficits with delayed onset. Front Neurosci 8:47
Kaizaki A et al (2013) Celecoxib reduces brain dopaminergic neuronal dysfunction, and improves sensorimotor behavioral performance in neonatal rats exposed to systemic lipopolysaccharide. J Neuroinflammation 10:45
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Magistretti PJ, Allaman I (2013) Brain energy metabolism. In: Neuroscience in the 21st Century. Springer, pp 1591–1620
Morin C, Zini R, Tillement J-P (2003) Anoxia–reoxygenation-induced cytochrome c and cardiolipin release from rat brain mitochondria. Biochem Biophys Res Commun 307:477–482
Puka-Sundvall M et al (2000) Impairment of mitochondrial respiration after cerebral hypoxia–ischemia in immature rats: relationship to activation of caspase-3 and neuronal injury. Dev Brain Res 125:43–50
Reddy NR, Krishnamurthy S, Chourasia TK, Kumar A, Joy KP (2011) Glutamate antagonism fails to reverse mitochondrial dysfunction in late phase of experimental neonatal asphyxia in rats. Neurochem Int 58:582–590
Rosenberg AA, Parks JK, Murdaugh E, Parker WD Jr (1989) Mitochondrial function after asphyxia in newborn lambs Stroke. J Cereb Circ 20:674–679
Singh IN, Sullivan PG, Deng Y, Mbye LH, Hall ED (2006) Time course of post-traumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: implications for neuroprotective therapy. J Cereb Blood Flow Metab: Off J Int Soc Cereb Blood Flow Metab 26:1407–1418
Singh IN, Sullivan PG, Hall ED (2007) Peroxynitrite-mediated oxidative damage to brain mitochondria: protective effects of peroxynitrite scavengers. J Neurosci Res 85:2216–2223
Strata F, Coq JO, Byl N, Merzenich MM (2004) Effects of sensorimotor restriction and anoxia on gait and motor cortex organization: Implications for a rodent model of cerebral palsy. Neuroscience 129:141–156
Sun D, Gilboe DD (1994) Ischemia‐induced changes in cerebral mitochondrial free fatty acids, phospholipids, and respiration in the rat. J Neurochem 62:1921–1928
Takada SH, Sampaio CAG, Allemandi W, Ito PH, Takase LF, Nogueira MI (2011) A modified rat model of neonatal anoxia: Development and evaluation by pulseoximetry, arterial gasometry and Fos immunoreactivity vol 198
Acknowledgments
SK is thankful to Department of Biotechnology (DBT), New Delhi, India for assistance in terms of research grant [102/IFD/SAN/4654/2011-2012].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Samaiya, P.K., Krishnamurthy, S. Characterization of mitochondrial bioenergetics in neonatal anoxic model of rats. J Bioenerg Biomembr 47, 217–222 (2015). https://doi.org/10.1007/s10863-015-9603-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-015-9603-2