Abstract
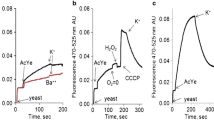
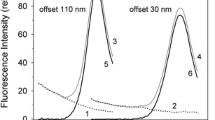
Recently we introduced a fluorescent probe technique that makes possible to convert changes of equilibrium fluorescence spectra of 3,3’-dipropylthiadicarbocyanine, diS-C3(3), measured in yeast cell suspensions under defined conditions into underlying membrane potential differences, scaled in millivolts (Plasek et al. in J Bioenerg Biomembr 44: 559–569, 2012). The results presented in this paper disclose measurements of real early changes of plasma membrane potential induced by the increase of extracellular K+, Na+ and H+ concentration in S. cerevisiae with and without added glucose as energy source. Whereas the wild type and the ∆tok1 mutant cells exhibited similar depolarization curves, mutant cells lacking the two Trk1,2 potassium transporters revealed a significantly decreased membrane depolarization by K+, particularly at lower extracellular potassium concentration [K+]out. In the absence of external energy source plasma membrane depolarization by K+ was almost linear. In the presence of glucose the depolarization curves exhibited an exponential character with increasing [K+]out. The plasma membrane depolarization by Na+ was independent from the presence of Trk1,2 transporters. Contrary to K+, Na+ depolarized the plasma membrane stronger in the presence of glucose than in its absence. The pH induced depolarization exhibited a fairly linear relationship between the membrane potential and the pHo of cell suspensions, both in the wild type and the Δtrk1,2 mutant strains, when cells were energized by glucose. In the absence of glucose the depolarization curves showed a biphasic character with enhanced depolarization at lower pHo values.
Similar content being viewed by others
References
Arino J, Ramos J, Sychrova H (2010) Alkali metal cation transport and homeostasis in yeasts. Microbiol Mol Biol Rev 74:95–120
Banuelos MA, Sychrova H, Bleykasten-Grosshans C, Souciet JL, Potier S (1998) The Nha1 antiporter of Saccharomyces cerevisiae mediates sodium and potassium efflux. Microbiology 144:2749–2758
Bertl A, Bihler H, Reid JD, Kettner C, Slayman CL (1998) Physiological characterization of the yeast plasma membrane outward rectifying K+ channel, DUK1 (TOK1), in situ. J Membr Biol 162:67–80
Brachmann CB, Davies A, Cost GJ, Caputo E, Li JC, Hieter P, Boeke JD (1998) Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14:115–132
Brett CL, Tukaye DN, Mukherjee S, Rao RJ (2005) The yeast endosomal Na+(K+)/H+ exchanger Nhx1 regulates cellular pH to control vesicle trafficking. Mol Biol Cell 16:1396–1405
Corratge-Faillie C, Jabnoune M, Zimmermann S, Very AA, Fizames C, Sentenac H (2010) Potassium and sodium transport in non-animal cells: the Trk/Ktr/HKT transporter family. Cell Mol Life Sci 67:2511–2532
Evans HJ, Wildes RA (1971) Potassium and its role in enzyme activation. In: Proceedings of the 8th Colloquium of the International Potash Institute. International Potash Institute, Berne, pp 13–39
Gášková D, Brodská B, Heřman P, Večeř J, Malínský J, Sigler K, Benada O, Plášek J (1998) Fluorescent probing of membrane potential in walled cells: diS-C-3(3) assay in Saccharomyces cerevisiae. Yeast 14:1189–1197
Jaffe LF (1974) The interpretation of voltage-concentration relations. J Theor Biol 48:11–18
Johnston D, Wu SM-S (1995) Foundations of cellular neurophysiology. MIT Press, Cambridge, Chapt. 2
Ke RA, Ingram PJ, Haynes K (2013) An integrative model of ion regulation in yeast. PLoS Comput Biol 9:e1002879. doi:10.1371/journal.pcbi.1002879
Kotyk A, Janacek K (1977) Membrane transport: an interdisciplinary approach. Plenum Press, New York
Leigh RA, Jones RGW (1984) A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant cell. New Phytol 97:1–13
Madrid R, Gomez MJ, Ramos J, Rodriguez-Navarro A (1998) Ectopic potassium uptake in trk1 trk2 mutants of Saccharomyces cerevisiae correlates with a highly hyperpolarized membrane potential. J Biol Chem 273:14838–14844
Maresova L, Urbankova E, Gaskova D, Sychrova H (2006) Measurements of plasma membrane potential changes in Saccharomyces cerevisiae cells reveal the importance of the Tok1 channel in membrane potential maintenance. FEMS Yeast Res 6:1039–1046
Maresova L, Muend S, Zhang YQ, Sychrova H, Rao R (2009) Membrane hyperpolarization drives cation influx and fungicidal activity of amiodarone. J Biol Chem 284:2795–2802
Miesenbock G, De Angelis DA, Rothman JE (1998) Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394:192–195
Mulet JM, Leube MP, Kron SJ, Rios G, Fink GR, Serrano R (1999) A novel mechanism of ion homeostasis and salt tolerance in yeast: the Hal4 and Hal5 protein kinases modulate the Trk1-Trk2 potassium transporter. Mol Cell Biol 19:3328–3337
Nakayama H, Yoshida K, Shinmyo A (2004) Yeast plasma membrane Ena1p ATPase alters alkali-cation homeostasis and confers increased salt tolerance in tobacco cultured cells. Biotechnol Bioeng 85:776–789
Navarrete C, Petrezselyova S, Barreto L, Martinez JL, Zahradka J, Arino J, Sychrova H, Ramos J (2010) Lack of main K plus uptake systems in Saccharomyces cerevisiae cells affects yeast performance in both potassium-sufficient and potassium-limiting conditions. FEMS Yeast Res 10:508–517
Offner FF (1991) Ion flow through membranes the resting potential of cells. J Membr Biol 123:171–182
Pena A, Uribe S, Pardo JP, Borbolla M (1984) The use of a cyanine dye in measuring membrane potential in yeast. Arch Biochem Biophys 231:217–225
Pena A, Sanchez NS, Calahorra M (2010) Estimation of the electric plasma membrane potential difference in yeast with fluorescent dyes: comparative study of methods. J Bioenerg Biomembr 42:419–432
Petrezselyova S, Zahradka J, Sychrova H (2010) Saccharomyces cerevisiae BY4741 and W303-1A laboratory strains differ in salt tolerance. Fungal Biol 114:144–150
Petrezselyova S, Ramos J, Sychrova H (2011) Trk2 transporter is a relevant player in K+ supply and plasma-membrane potential control in Saccharomyces cerevisiae. Folia Microbiol 56:23–28
Plášek J, Gášková D, Lichtenberg-Fraté H, Ludwig J, Höfer M (2012) Monitoring of real changes of plasma membrane potential by diS-C3(3) fluorescence in yeast cell suspensions. J Bioenerg Biomembr 44:559–569
Rodriguez-Navarro A (2000) Potassium transport in fungi and plants. Biochim Biophys Acta 1469:1–30
Serrano R (1991) Transport across yeast vacuolar and plasma membranes. In: Broach JR, Jones EW, Prigle JR (eds) The molecular and cellular biology of the yeast Saccharomyces. Genomic dynamics, protein synthesis and energetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, pp 523–585
Serrano R, Mulet JM, Rios G, Marquez JA, de Larrinoa IF, Leube MP, Mendizabal I, Pascual-Ahuir A, Proft M, Ros R, Montesinos C (1999) A glimpse of the mechanisms of ion homeostasis during salt stress. J Exp Bot 50:1023–1036
Sunder S, Singh AJ, Gill S, Singh B (1996) Regulation of intracellular level of Na+, K+ and glycerol in Saccharomyces cerevisiae under osmotic stress. Mol Cell Biochem 158:121–124
Zahradka J, Sychrova H (2012) Plasma-membrane hyperpolarization diminishes the cation efflux via Nha1 antiporter and Ena ATPase under potassium-limiting conditions. FEMS Yeast Res 12:439–446
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Plášek, J., Gášková, D., Ludwig, J. et al. Early changes in membrane potential of Saccharomyces cerevisiae induced by varying extracellular K+, Na+ or H+ concentrations. J Bioenerg Biomembr 45, 561–568 (2013). https://doi.org/10.1007/s10863-013-9528-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-013-9528-6