Abstract
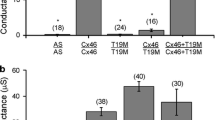
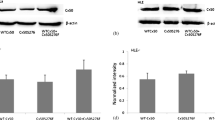
Connexin46 (Cx46), together with Cx50, forms gap junction channels between lens fibers and participates in the lens pump-leak system, which is essential for the homeostasis of this avascular organ. Mutations in Cx50 and Cx46 correlate with cataracts, but the functional relationship between the mutations and cataract formation is not always clear. Recently, it was found that a mutation at the third position of hCx46 that substituted an aspartic acid residue with a tyrosine residue (hCx46D3Y) caused an autosomal dominant zonular pulverulent cataract. We expressed EGFP-labeled hCx46wt and hCx46D3Y in HeLa cells and found that the mutation did not affect the formation of gap junction plaques. Dye transfer experiments using Lucifer Yellow (LY) and ethidium bromide (EthBr) showed an increased degree of dye coupling between the cell pairs expressing hCx46D3Y in comparison to the cell pairs expressing hCx46wt. In Xenopus oocytes, two-electrode voltage-clamp experiments revealed that hCx46wt formed voltage-sensitive hemichannels. This was not observed in the oocytes expressing hCx46D3Y. The replacement of the aspartic acid residue at the third position by another negatively charged residue, glutamic acid, to generate the mutant hCx46D3E, restored the voltage sensitivity of the resultant hemichannels. Moreover, HeLa cell pairs expressing hCx46D3E and hCx46wt showed a similar degree of dye coupling. These results indicate that the negatively charged aspartic acid residue at the third position of the N-terminus of hCx46 could be involved in the determination of the degree of metabolite cell-to-cell coupling and is essential for the voltage sensitivity of the hCx46 hemichannels.
Similar content being viewed by others
References
Abbaci M, Barberi-Heyob M, Blondel W, Guillemin F, Didelon J (2008) Advantages and limitations of commonly used methods to assay the molecular permeability of gap junctional intercellular communication. Biotechniques 45:33–62
Addison PK, Berry V, Holden KR, Espinal D, Rivera B, Su H, Srivastava AK, Bhattacharya SS (2006) A novel mutation in the connexin 46 gene (GJA3) causes autosomal dominant zonular pulverulent cataract in a Hispanic family. Mol Vis 12:791–795
Arora A, Minogue PJ, Liu X, Addison PK, Russel-Eggitt I, Webster AR, Hunt DM, Ebihara L, Beyer EC, Berthoud VM, Moore AT (2008) A novel connexin50 mutation associated with congenital nuclear pulverulent cataracts. J Med Genet 45:155–160
Arora, A., Minogue, P.J., Liu, X., Reddy, M.A., Ainsworth, J.R., Bhattacharya, S.S., Webster, A.R., Hunt, D.M., Ebihara, L., Moore, A.T., Beyer, E.C. and Berthoud, V.M. (2006) A novel GJA8 mutation is associated with autosomal dominant lamellar pulverulent cataract: further evidence for gap junction dysfunction in human cataract. J Med Genet. 43:e2 (http://www.jmedgenet.com/cgi/content/full/43/1/e2)
Berthoud VM, Beyer EC (2009) Oxidative stress, lens gap junctions, and cataracts. Antioxid Redox Signal 11:339–353
Berthoud VM, Minogue PJ, Guo J, Williamson EK, Xu X, Ebihara L, Beyer EC (2003) Loss of function and impaired degradation of a cataract-associated mutant connexin50. Eur J Cell Biol 82:209–221
Beyer EC, Lipkind GM, Kyle JW, Berthoud VM (2012) Structural organization of intercellular channels II. Amino terminal domain of the connexins: sequence, functional roles, and structure. Biochim Biophys Acta 1818:1823–1830
Donaldson P, Kistler J, Mathias RT (2001) Molecular solutions to mammalian lens transparency. News Physiol Sci 16:118–123
Dong L, Liu X, Li H, Vertel BM, Ebihara L (2006) Role of the N-terminus in permeability of chicken connexin45.6 gap junctional channels. J Physiol 576:787–799
Ebihara, L., Berthoud, V.M. and Beyer, E.C. (1995) Distinct behavior of connexin56 and connexin46 gap junctional channels can be predicted from the behavior of their hemi-gap-junctional channels. Biophys J.68:1796-1803
Elfgang C, Eckert R, Lichtenberg-Frate H, Butterweck A, Traub O, Klein RA, Hulser DF, Willecke K (1995) Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J Cell Biol 129:805–817
Falk MM, Kumar NM, Gilula NB (1994) Membrane insertion of gap junction connexins: polytopic channel forming membrane proteins. J Cell Biol 127:343–355
Gerido DA, White TW (2004) Connexin disorders of the ear, skin, and lens. Biochim Biophys Acta 1662:159–170
Gonzalez D, Gomez-Hernandez JM, Barrio LC (2007) Molecular basis of voltage dependence of connexin channels: an integrative appraisal. Prog Biophys Mol Biol 94:66–106
Goodenough DA (1992) The crystalline lens. A system networked by gap junctional intercellular communication. Semin Cell Biol 3:49–58
Hansen L, Yao W, Eiberg H, Funding M, Riise R, Kjaer KW, Hejtmancik JF, Rosenberg T (2006) The congenital “ant-egg” cataract phenotype is caused by a missense mutation in connexin46. Mol Vis 12:1033–1039
Krogh A, Larsson B, von Heijne G, Sonnhammer EL (2001) Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 305:567–580
Kyle JW, Berthoud VM, Kurutz J, Minogue PJ, Greenspan M, Hanck DA, Beyer EC (2009) The N terminus of connexin37 contains an alpha-helix that is required for channel function. J Biol Chem 284:20418–20427
Kyle JW, Minogue PJ, Thomas BC, Domowicz DA, Berthoud VM, Hanck DA, Beyer EC (2008) An intact connexin N-terminus is required for function but not gap junction formation. J Cell Sci 121:2744–2750
Lichtenstein A, Gaietta GM, Deerinck TJ, Crum J, Sosinsky GE, Beyer EC, Berthoud VM (2009) The cytoplasmic accumulations of the cataract-associated mutant, Connexin50P88S, are long-lived and form in the endoplasmic reticulum. Exp Eye Res 88:600–609
Maeda S, Nakagawa S, Suga M, Yamashita E, Oshima A, Fujiyoshi Y, Tsukihara T (2009) Structure of the connexin 26 gap junction channel at 3.5 A resolution. Nature 458:597–602
Maeda S, Tsukihara T (2011) Structure of the gap junction channel and its implications for its biological functions. Cell Mol Life Sci 68:1115–1129
Mathias RT, Rae JL, Baldo GJ (1997) Physiological properties of the normal lens. Physiol Rev 77:21–50
Mathias RT, White TW, Gong X (2010) Lens gap junctions in growth, differentiation, and homeostasis. Physiol Rev 90:179–206
Minogue PJ, Liu X, Ebihara L, Beyer EC, Berthoud VM (2005) An aberrant sequence in a connexin46 mutant underlies congenital cataracts. J Biol Chem 280:40788–40795
Minogue PJ, Tong JJ, Arora A, Russell-Eggitt I, Hunt DM, Moore AT, Ebihara L, Beyer EC, Berthoud VM (2009) A mutant connexin50 with enhanced hemichannel function leads to cell death. Invest Ophthalmol Vis Sci 50:5837–5845
Oh S, Abrams CK, Verselis VK, Bargiello TA (2000) Stoichiometry of transjunctional voltage-gating polarity reversal by a negative charge substitution in the amino terminus of a connexin32 chimera. J Gen Physiol 116:13–31
Oh S, Rivkin S, Tang Q, Verselis VK, Bargiello TA (2004) Determinants of gating polarity of a connexin 32 hemichannel. Biophys J 87:912–928
Oh S, Rubin JB, Bennett MV, Verselis VK, Bargiello TA (1999) Molecular determinants of electrical rectification of single channel conductance in gap junctions formed by connexins 26 and 32. J Gen Physiol 114:339–364
Pal JD, Liu X, Mackay D, Shiels A, Berthoud VM, Beyer EC, Ebihara L (2000) Connexin46 mutations linked to congenital cataract show loss of gap junction channel function. Am J Physiol Cell Physiol 279:C596–C602
Paul DL, Ebihara L, Takemoto LJ, Swenson KI, Goodenough DA (1991) Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J Cell Biol 115:1077–1089
Purnick PE, Benjamin DC, Verselis VK, Bargiello TA, Dowd TL (2000a) Structure of the amino terminus of a gap junction protein. Arch Biochem Biophys 381:181–190
Purnick PE, Oh S, Abrams CK, Verselis VK, Bargiello TA (2000b) Reversal of the gating polarity of gap junctions by negative charge substitutions in the N-terminus of connexin 32. Biophys J 79:2403–2415
Sohl G, Willecke K (2004) Gap junctions and the connexin protein family. Cardiovasc Res 62:228–232
Srinivas M, Kronengold J, Bukauskas FF, Bargiello TA, Verselis VK (2005) Correlative studies of gating in Cx46 and Cx50 hemichannels and gap junction channels. Biophys J 88:1725–1739
Thomas BC, Minogue PJ, Valiunas V, Kanaporis G, Brink PR, Berthoud VM, Beyer EC (2008) Cataracts are caused by alterations of a critical N-terminal positive charge in connexin50. Invest Ophthalmol Vis Sci 49:2549–2556
Tong JJ, Liu X, Dong L, Ebihara L (2004) Exchange of gating properties between rat cx46 and chicken cx45.6. Biophys J 87:2397–2406
Verselis VK, Ginter CS, Bargiello TA (1994) Opposite voltage gating polarities of two closely related connexins. Nature 368:348–351
Walter WJ, Zeilinger C, Bintig W, Kolb HA, Ngezahayo A (2008) Phosphorylation in the C-terminus of the rat connexin46 (rCx46) and regulation of the conducting activity of the formed connexons. J Bioenerg Biomembr 40:397–405
White TW, Bruzzone R (2000) Intercellular communication in the eye: clarifying the need for connexin diversity. Brain Res Brain Res Rev 32:130–137
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schlingmann, B., Schadzek, P., Busko, S. et al. Cataract-associated D3Y mutation of human connexin46 (hCx46) increases the dye coupling of gap junction channels and suppresses the voltage sensitivity of hemichannels. J Bioenerg Biomembr 44, 607–614 (2012). https://doi.org/10.1007/s10863-012-9461-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-012-9461-0