Abstract
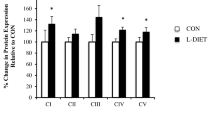
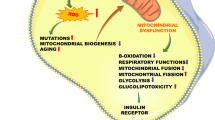
In this work we review recent findings that explain how mitochondrial bioenergetic functions and redox state respond to a hyperlipidemic in vivo environment and may contribute to the maintenance of a normal metabolic phenotype. The experimental model utilized to evidence these adaptive mechanisms is especially useful for these studies since it exhibits genetic hypertriglyceridemia and avoids complications introduced by high fat diets. Liver from hypertrigliceridemic (HTG) mice have a greater content of glycerolipids together with increased mitochondrial free fatty acid oxidation. HTG liver mitochondria have a higher resting respiration rate but normal oxidative phosphorylation efficiency. This is achieved by higher activity of the mitochondrial potassium channel sensitive to ATP (mitoKATP). The mild uncoupling mediated by mitoKATP accelerates respiration rates and reduces reactive oxygen species generation. Although this response is not sufficient to inhibit lipid induced extra-mitochondrial oxidative stress in whole liver cells it avoids amplification of this redox imbalance. Furthermore, higher mitoKATP activity increases liver, brain and whole body metabolic rates. These mitochondrial adaptations may explain why these HTG mice do not develop insulin resistance and obesity even under a severe hyperlipidemic state. On the contrary, when long term high fat diets are employed, insulin resistance, fatty liver and obesity develop and mitochondrial adaptations are inefficient to counteract energy and redox imbalances.
Similar content being viewed by others
References
Aalto-Setala K, Fisher EA, Chen X, Chajek-Shaul T, Hayek T, Zechner R, Walsh A, Ramakrishnan R, Ginsberg HN, Breslow JL (1992) J Clin Invest 90:1889–1900
Alberici LC, Oliveira HC, Bighetti EJ, de Faria EC, Degaspari GR, Souza CT, Vercesi AE (2003) J Bioenerg Biomembr 35:451–457
Alberici LC, Oliveira HC, Patrício PR, Kowaltowski AJ, Vercesi AE (2006) Gastroenterology 131:1228–1234
Alberici LC, Oliveira HC, Paim BA, Mantello CC, Augusto AC, Zecchin KG, Gurgueira SA, Kowaltowski AJ, Vercesi AE (2009) Free Radic Biol Med 47:1432–1439
Amaral ME, Oliveira HC, Carneiro EM, Delghingaro-Augusto V, Vieira EC, Berti JA, Boschero AC (2002) Horm Metab Res 34:21–26
Boveris A (1977) Adv Exp Med Biol 78:67–82
Cardoso AR, Cabral-Costa JV, Kowaltowski AJ (2010) J Bioenerg Biomembr 42:245–253
Cighetti G, Bortone L, Sala S, Allevi P (2001) Arch Biochem Biophys 389:195–200
Clapham JC, Arch JR, Chapman H, Haynes A, Lister C, Moore GB, Piercy V, Carter SA, Lehner I, Smith SA, Beeley LJ, Godden RJ, Herrity N, Skehel M, Changani KK, Hockings PD, Reid DG, Squires SM, Hatcher J, Trail B, Latcham J, Rastan S, Harper AJ, Cadenas S, Buckingham JA, Brand MD, Abuin A (2000) Nature 406:415–418
Després JP, Lemieux I (2006) Nature 444:881–887
Facundo HT, de Paula JG, Kowaltowski AJ (2007) Free Radic Biol Med 42:1039–1048
Fornazari M, de Paula JG, Castilho RF, Kowaltowski AJ (2008) J Neurosci Res 86:1548–1556
Garlid KD, Paucek P (2003) Biochim Biophys Acta 1606:23–41
Garlid KD, Jaburek M, Jezek P, Varecha M (2000) Biochim Biophys Acta 1459:383–389
Greenberg JA (1999) Med Hypotheses 52:15–22
Greenberg JA, Boozer CN (2000) Mech Ageing Dev 113:37–48
Grundy SM, Brewer HB Jr, Cleeman JI, Smith SC Jr, Lenfant C, American Heart Association, National Heart, Lung, and Blood Institute (2004) Circulation 109:433–438
Hesselink MK, Mensink M, Schrauwen P (2003) Obes Res 11:1429–1443
Ito Y, Azrolan N, O’Connell A, Walsh A, Breslow JL (1990) Science 249:790–793
Jezek P, Garlid KD (1998) Int J Biochem Cell Biol 30:1163–1168
Kontani Y, Wang Y, Kimura K, Inokuma KI, Saito M, Suzuki-Miura T, Wang Z, Sato Y, Mori N, Yamashita H (2005) Aging Cell 4:147–155
Kopecky J, Clarke G, Enerback S, Spiegelman B, Kozak LP (1995) J Clin Invest 96:2914–2923
Krylova IB, Kachaeva EV, Rodionova OM, Negoda AE, Evdokimova NR, Balina MI, Sapronov NS, Mironova GD (2006) Exp Gerontol 41:697–703
Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR (2000) J Clin Invest 105:1067–1075
Meilhac O, Zhou M, Santanam N, Parthasarathy S (2000) J Lipid Res 41:1205–1213
Nicholls DG (1976) FEBS Lett 61:103–110
Nishikawa T, Kukidome D, Sonoda K, Fujisawa K, Matsuhisa T, Motoshima H, Matsumura T, Araki E (2007) Diabetes Res Clin Pract 77:161–164
Reaven GM, Mondon CE, Chen YD, Breslow JL (1994) J Lipid Res 820–824.
Rosen ED, Spiegelman BM (2006) Nature 444:847–853
Salerno AG, Silva TR, Amaral ME, Alberici LC, Bonfleur ML, Patrício PR, Francesconi EP, Grassi-Kassisse DM, Vercesi AE, Boschero AC, Oliveira HC (2007) Int J Obes (Lond) 31:1586–1595
Samartsev VN, Mokhova EN (1997) Biochem Mol Biol Int 42:29–34
Schönfeld P, Wojtczak L (2008) Free Radic Biol Med 45:231–241
Schrauwen P, Hesselink MK (2004) Proc Nutr Soc 63:287–292
Skulachev VP (1991) FEBS Lett 294:158–162
Sperl W, Skladal D, Gnaiger E, Wyss M, Mayr U, Hager J, Gellerich FN (1997) Mol Cell Biochem 174:71–78
Vercesi AE, Martins IS, Silva MAP, Leite HMF, Cuccovia IM, Chaimovich H (1995) Nature 375:24
Zhang DX, Chen YF, Campbell WB, Zou AP, Gross GJ, Li PL (2001) Circ Res 89:1177–1183
Zhang CM, Gu Y, Qing DN, Zhu JG, Zhu C, Zhang M, Guo XR (2010) Mol Biol Rep 37:3177–3182
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alberici, L.C., Vercesi, A.E. & Oliveira, H.C.F. Mitochondrial energy metabolism and redox responses to hypertriglyceridemia. J Bioenerg Biomembr 43, 19–23 (2011). https://doi.org/10.1007/s10863-011-9326-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-011-9326-y